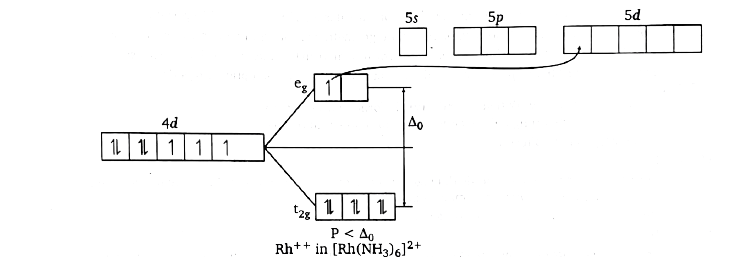
Crystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex.
Q. In which of the following complex transition of electron occurs from one shell to other shell of central metal.
[Fe(H2O)5(NO)]2+
[Co(H2O)6]2+
[Rh(NH3)6]2+
[Ni(CN)6]4−
The correct Answer is:C
Topper's Solved these Questions
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:ONE OR MORE ANSWERS IS/ARE CORRECT
Explore 71 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:MATCH THE COLUMN
Explore 17 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:LEVEL 2
Explore 143 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (BASIC)Exercise:SUBJECTIVE PROBLEMS
Explore 49 Videos
- d-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:d-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 6 Videos
Similar Questions
Consider the following two reactions:
Cd2+(aq.)+4CH3NH2K1−−→'A',ΔG∘1
Cd2+(aq.)+2H2NCH2CH2NH2K2−−→'B',ΔG∘2. According to given information the correct statement(s) is/are:
View SolutionMatch the following columns

View SolutionWhich of the following ligand does not as π−acid ligand?
View SolutionConsider following compounds A to E :
(A) XeFn (B)XeF+(n+1) (C)XeF−(n+1) (D)XeF(n+2)
(E ) XeF2−(n+4),
If value of n is 4, then calculate value of p÷q here, 'p' is total number of bond pair and 'q' is total number of lone pair on central atoms of compounds (A) to (E ).
View SolutionCalculate the magnetic moment of a high-spin octahedral complex that has six electrons in 3d-orbitals
View SolutionWhich of the following d-electron distribution of the diamagnetic complex [Ni(CN)]^(2-) is possible according to the crystal field theory ?
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex. Q. Which of the following complex is high spin?
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex. Q. In which of the following complex transition of electron occurs from one shell to other shell of central metal.
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex.
Q. Which of the following hydrated complex ion has high intensity colour in aqueous solution.
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex.
Q. Which of the following complex is high spin?
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex.
Q. In which of the following complex transition of electron occurs from one shell to other shell of central metal.
View SolutionCrystal field theory provides correct electronic distribution of central metal under surrounding ligannd field, hence it clearly explains magnetic moment, colour of a complex.
Q. Which of the following hydrated complex ion has high intensity colour in aqueous solution.
View Solutionকেন্দ্রীয় ধাতব পরমাণুতে d-ইলেকট্রন নেই এমন জটিল আয়ন কোন্টি?
View SolutionThe complex ion which has no 'd'-electrons in the central metal atom is
View SolutionThe complex ion which has no 'd' electrons in the central metal atom is _________.
View SolutionThe complex ion which has no. ‘d’ electrons in the central metal atom is:
View Solution