When degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
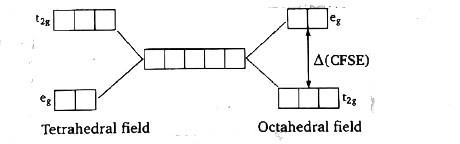
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. Cr3+ form four complexes with four different ligands which are [Cr(Cl)6]3−,[Cr(H2O)6]3+,
[Cr(NH3)6]3+and[Cr(CN)6]3−, the order of CFSE (Δ0) in these complexes in the order:
[CrCl6]3+and[Cr(CN)6]3+=[Cr(NH3)6]3+=[Cr(CN)6]3−
[CrCl6]3−<[Cr(H2O)6]3+<[Cr(NH3)6]3+<[Cr(CN)6]3−
[CrCl6]3−>[Cr(H2O)6]3+>[Cr(NH3)6]3+>[Cr(CN)6]3−
[CrCl6]3−<[Cr(H2O)6]3+=[Cr(NH3)6]3+<[Cr(CN)6]3−
The correct Answer is:B
Step by step video solution for When degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Cr^(3+) form four complexes with four different ligands which are [Cr(Cl)_(6)]^(3-), [Cr(H_(2)O)_(6)]^(3+), [Cr(NH_(3))_(6)]^(3+) and [Cr(CN)_(6)]^(3-), the order of CFSE (Delta_(0)) in these complexes in the order: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:ONE OR MORE ANSWERS IS/ARE CORRECT
Explore 71 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:MATCH THE COLUMN
Explore 17 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWAL ENGLISHChapter:CO-ORDINATION COMPOUNDSExercise:LEVEL 2
Explore 143 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (BASIC)Exercise:SUBJECTIVE PROBLEMS
Explore 49 Videos
- d-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:d-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 6 Videos
Similar Questions
Consider the following two reactions:
Cd2+(aq.)+4CH3NH2K1−−→'A',ΔG∘1
Cd2+(aq.)+2H2NCH2CH2NH2K2−−→'B',ΔG∘2. According to given information the correct statement(s) is/are:
View SolutionMatch the following columns

View SolutionWhich of the following ligand does not as π−acid ligand?
View SolutionConsider following compounds A to E :
(A) XeFn (B)XeF+(n+1) (C)XeF−(n+1) (D)XeF(n+2)
(E ) XeF2−(n+4),
If value of n is 4, then calculate value of p÷q here, 'p' is total number of bond pair and 'q' is total number of lone pair on central atoms of compounds (A) to (E ).
View SolutionCalculate the magnetic moment of a high-spin octahedral complex that has six electrons in 3d-orbitals
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Cr^(3+) form four complexes with four different ligands which are [Cr(Cl)_(6)]^(3-), [Cr(H_(2)O)_(6)]^(3+) , [Cr(NH_(3))_(6)]^(3+) and [Cr(CN)_(6)]^(3-) , the order of CFSE (Delta_(0)) in these complexes in the order:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
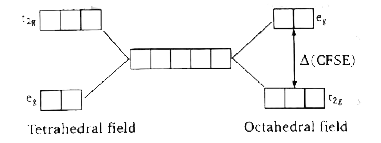
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. The d-orbitals, which are stabillized in an octahedral magnetic field, are:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
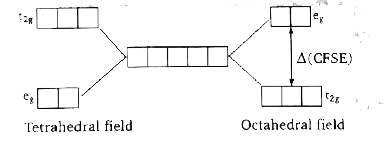
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. For an octahedral complex, which of the followin d-electron configuration will give maximum CFSE?
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Ti^(3+)(aq) . is purple while Ti^(4+)(aq) . is colourless because:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. Which of the following is correct arrangement of ligand in terms of the Dq values of their complexes with any particularr 'hard' metal ion:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
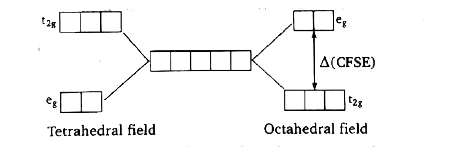
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. The extent of crystal field splitting in octahedral complexes of the given metal with particular weak field ligand are:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:

Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. Cr3+ form four complexes with four different ligands which are [Cr(Cl)6]3−,[Cr(H2O)6]3+,
[Cr(NH3)6]3+and[Cr(CN)6]3−, the order of CFSE (Δ0) in these complexes in the order:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t_(2g)(d_(xy),d_(yz),d_(xz)) and e_(g) (d_(x^(2))-d_(x^(2)-y^(2)) are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as: Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Delta is about 4/9 times to Delta_(0) (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d^(0) and d^(10) configuration. Q. The d-orbitals, which are stabillized in an octahedral magnetic field, are:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:

Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. For an octahedral complex, which of the followin d-electron configuration will give maximum CFSE?
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
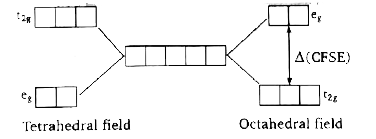
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. Ti3+(aq). is purple while Ti4+(aq). is colourless because:
View SolutionWhen degenerate d-orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneray is lost. The two set t2g(dxy,dyz,dxz)andeg(dx2−dx2−y2 are either stabilized or destabilized depending upon the nature of magnetic field. it can be expressed diagrammatically as:
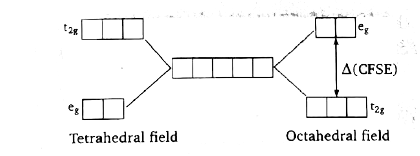
Value of CFSE depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, Δ is about 4/9 times to Δ0 (CFSE for octahedral complex). this energy lies in visible region and i.e., why electronic transition are responsible for colour. such transition are not possible with d0andd10 configuration.
Q. Which of the following is correct arrangement of ligand in terms of the Dq values of their complexes with any particularr 'hard' metal ion:
View Solution
