In polymeric (BeCl2)n, there are:
Three centre four-electron bonds
Three centre three-electron bonds
two centre three-electron bonds
two centre two-electron bonds
The correct Answer is:A
Step by step video solution for In polymeric (BeCl_(2))_(n), there are: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - Level 3
47 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - ONE OR MORE ANSWER IS/ARE CORRECT
66 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
3 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
Similar Questions
Dilute solutions of alkali metals in liquid NH3 are blue. It is the ammoniated electron which is responsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH3)x]+ as well as the ammoniated electrons, [e(NH3)y]−, values of x and y depend on the extent of solvation (by NH3). Dilute solutions are paramagnetic due to free electrons.
What happens if alkali metals is allowed to react with concentrated liquid ammonia?
View SolutionThe correct statement(s) is /are
View Solution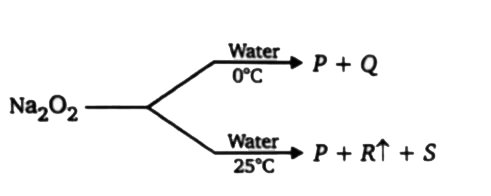
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionFind number of basic radicals among the following cations, which can form soluble complex on adding excess of NH4 solution.
Cd2+(aq.),Pb2+(aq.),Ni2+(aq.),Mn2+(aq.),Zn2+(aq.),Ag+(aq.),Hg2+(aq.),Fe3+(aq.),Mg2+(aq.)
View SolutionMatch the following columns

View SolutionIn anhydroous state BeCl2 is
View SolutionBeryllium chloride exists as polymeric chain having fromula (BeCl2)n. If a compound Kx(Be4Cl10) has finite chain, find the value of x.
View SolutionBeCl2+LiAlH4
View SolutionDraw the structures of (i) BeCl_(2) (vapour) (ii) BeCl_(2) (solid).
View SolutionBeCl_(2) is not isostructural with
View SolutionIn polymeric (BeCl2)n, there are:
View Solutionनिम्नलिखित की संरचना बताइए :
(a) BeCl2 (वाष्प), (b) BeCl2 (ठोस) ।
View SolutionDraw the structure of (i) BeCl2 (vapour) and (ii) BeCl2 (solid).
View SolutionBeCl2 is not isostructural with
View SolutionDraw the structure of (a) BeCl2(vapour) and (b) BeCl2 (solid).
View SolutionAqueous solution of BeCl_(2) is
View Solution

