Most metal oxide are thermally stable at temperature upto 1000∘C but the oxide of metals below hydrogen in the electrochemical series fairly. Thus HgOandAg2O decomposes on heating.
Q. Correct code for following thermal decomposition reaction(s) evolving gas having equal number of σ−andπ−bonds is :
(i)BeCO3δ−−→ (ii)ZnSO4δ−−−−−−−−→γ<800∘C
(iii)FeSO4300∘C−−−→ (iv)(NH4)2Cr2O7δ−−→
i, ii, iii
i, iii, iv
i, ii, iii and IV
All of these
The correct Answer is:A
To solve the problem, we need to analyze the thermal decomposition reactions given and identify the gases produced in each reaction. Then, we will determine which of these gases have equal numbers of sigma (σ) and pi (π) bonds.
Step 1: Analyze the Reactions
1. Reaction (i):
BeCO3Δ−→BeO+CO2
- Products: Beryllium oxide (BeO) and carbon dioxide (CO₂).
- Gas produced: CO₂.
2. Reaction (ii):
ZnSO4Δ<800∘C−−−−−−→ZnO+SO2+O2
- Products: Zinc oxide (ZnO), sulfur dioxide (SO₂), and oxygen (O₂).
- Gases produced: SO₂ and O₂.
3. Reaction (iii):
FeSO4300∘C−−−→Fe2O3+SO2+SO3
- Products: Ferric oxide (Fe₂O₃), sulfur dioxide (SO₂), and sulfur trioxide (SO₃).
- Gases produced: SO₂ and SO₃.
4. Reaction (iv):
(NH4)2Cr2O7Δ−→Cr2O3+N2+H2O
- Products: Chromium oxide (Cr₂O₃), nitrogen gas (N₂), and water (H₂O).
- Gas produced: N₂.
Step 2: Determine Sigma and Pi Bonds in Gases
1. Carbon Dioxide (CO₂):
- Structure: O=C=O
- Bonds: 2 σ bonds (one between C and each O) and 2 π bonds (one for each double bond).
- Equal σ and π bonds: Yes.
2. Sulfur Dioxide (SO₂):
- Structure: O=S=O
- Bonds: 2 σ bonds (one between S and each O) and 2 π bonds (one for each double bond).
- Equal σ and π bonds: Yes.
3. Oxygen (O₂):
- Structure: O=O
- Bonds: 1 σ bond and 1 π bond.
- Equal σ and π bonds: Yes.
4. Sulfur Trioxide (SO₃):
- Structure: O=S=O (with one additional O connected to S through a double bond).
- Bonds: 3 σ bonds and 3 π bonds.
- Equal σ and π bonds: Yes.
5. Nitrogen (N₂):
- Structure: N≡N
- Bonds: 1 σ bond and 2 π bonds.
- Equal σ and π bonds: No.
Step 3: Conclusion
The gases produced in reactions (i), (ii), (iii), and (iv) are:
- (i) CO₂
- (ii) SO₂, O₂
- (iii) SO₂, SO₃
- (iv) N₂
The gases with equal numbers of σ and π bonds are:
- CO₂
- SO₂
- O₂
- SO₃
Thus, the correct reactions that produce gases with equal numbers of σ and π bonds are:
- Reaction (i): CO₂
- Reaction (ii): SO₂, O₂
- Reaction (iii): SO₂, SO₃
Final Answer
The correct code for the reactions is: (i), (ii), (iii).
BeCO3Δ−→BeO+CO2
- Products: Beryllium oxide (BeO) and carbon dioxide (CO₂).
- Gas produced: CO₂.
ZnSO4Δ<800∘C−−−−−−→ZnO+SO2+O2
- Products: Zinc oxide (ZnO), sulfur dioxide (SO₂), and oxygen (O₂).
- Gases produced: SO₂ and O₂.
FeSO4300∘C−−−→Fe2O3+SO2+SO3
- Products: Ferric oxide (Fe₂O₃), sulfur dioxide (SO₂), and sulfur trioxide (SO₃).
- Gases produced: SO₂ and SO₃.
(NH4)2Cr2O7Δ−→Cr2O3+N2+H2O
- Products: Chromium oxide (Cr₂O₃), nitrogen gas (N₂), and water (H₂O).
- Gas produced: N₂.
- Structure: O=C=O
- Bonds: 2 σ bonds (one between C and each O) and 2 π bonds (one for each double bond).
- Equal σ and π bonds: Yes.
- Structure: O=S=O
- Bonds: 2 σ bonds (one between S and each O) and 2 π bonds (one for each double bond).
- Equal σ and π bonds: Yes.
- Structure: O=O
- Bonds: 1 σ bond and 1 π bond.
- Equal σ and π bonds: Yes.
- Structure: O=S=O (with one additional O connected to S through a double bond).
- Bonds: 3 σ bonds and 3 π bonds.
- Equal σ and π bonds: Yes.
- Structure: N≡N
- Bonds: 1 σ bond and 2 π bonds.
- Equal σ and π bonds: No.
- (i) CO₂
- (ii) SO₂, O₂
- (iii) SO₂, SO₃
- (iv) N₂
- CO₂
- SO₂
- O₂
- SO₃
- Reaction (i): CO₂
- Reaction (ii): SO₂, O₂
- Reaction (iii): SO₂, SO₃
The correct code for the reactions is: (i), (ii), (iii).
Topper's Solved these Questions
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - ONE OR MORE ANSWER IS/ARE CORRECT
66 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - MATCH THE COLUMN
6 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - Level 2
86 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
3 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
Similar Questions
Most metal oxide are thermally stable at temperature upto 1000∘C but the oxide of metals below hydrogen in the electrochemical series fairly. Thus HgOandAg2O decomposes on heating.
Q. Which of the following salt salt does not give NO2 gas on heating?
View SolutionMost metal oxide are thermally stable at temperature upto 1000∘C but the oxide of metals below hydrogen in the electrochemical series fairly. Thus HgOandAg2O decomposes on heating.
Q. Which of the following compound cannot be thermally decomposed even at high temperature?
View Solution
Knowledge Check
CH_(3)-underset(Br)underset(|)(CH)-CH_(2)-overset(O)overset(||)(C)-CH_(3)underset(Delta)overset(N_(2)H_O(4),overset(ϴ)(O)H)(to) Product:
A B
B C
C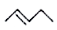 D
D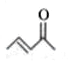
Consider the following sequence of reactions
CH3−O∣∣C−CH3Ba(OH)2−−−−−→AΔ−−→B↓NaBH4
The final product (C) is:A(CH3)2C(OH)CH2COCH3B(CH3)2C=CHCOCH3C(CH3)2CHCH2CHOHCH3D(CH3)2C=CHCHOHCH3
Identify A to F :
ALiAlH4−−−→C2H5OHPBr3−−→BKCN−−→CD−−→C3H7NH2HNO2−−−→E[O]−−−−−−−−−−→K2Cr2O7/H+F
View SolutionComplete and balance the following equation:
(c). KMnO4△−−−→200∘C
(iv). MnOɵ4+AsOɵ3+H⊕→
(v). K2Cr2O7+NH4Cl−−−→
View SolutionIdentify unknown compounds A to E in the following series of chemical reactions.
(i)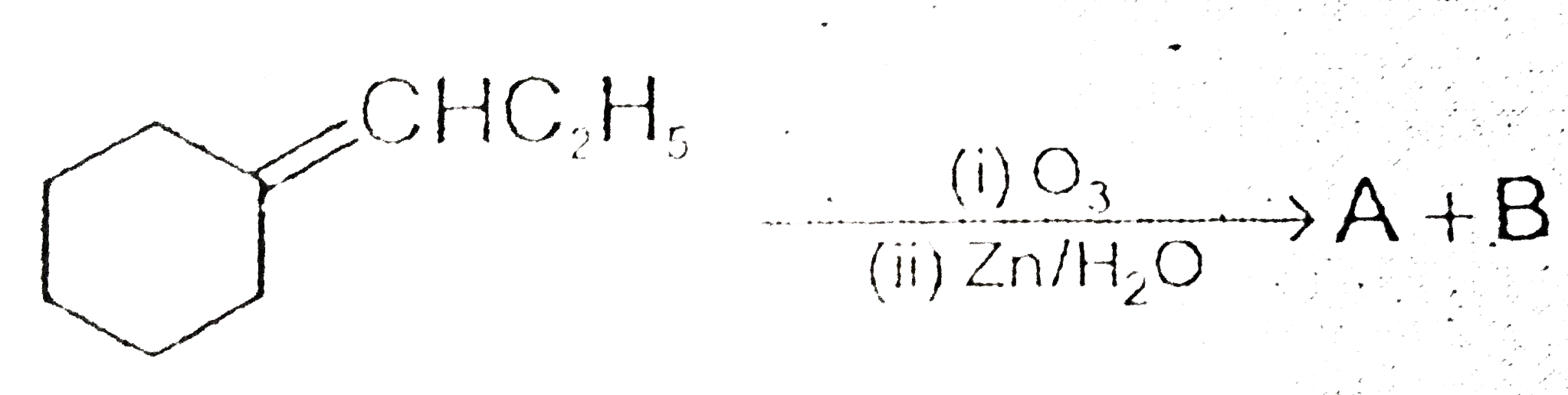
(ii) A+Bdil NaOH−−−−→C+H2O
(iii) C(i)O3−−−−−−−→(ii)Zn/H2OA+D
(iv) DH2/Ni−−−→ΔE
View SolutionIn the given reaction sequences the final product 'C' is
CH3−CH2−OH(i)KMnO4/−OH/Δ−−−−−−−−−−−−−−−→(ii)H2O+(A)(i)SOCl2−−−−−−−−→(ii)NH3(B)Br2/KOH−−−−−−→(C)
View SolutionWhich of the following is not a disproprotionation reaction?
I. NH4NO3Δ−−→N2O+H2O
II. P4Δ−−→PH3+HPOө2
III. PCl5Δ−−→PCl3+Cl2
IV. IOө3+Iө→I2
View SolutionThe major product (Y) in the following reactions is:
CH3−CH3∣CH−C≡CHHgSO4,H2SO4−−−−−−−−→H2OX(i)C2H5MgBr,H2O−−−−−−−−−−−−−→(ii)Conc,H2SO4/ΔY
View Solution

