Identify the correct statement(s):
Sodium carbonate on heating evolves carbon dioxide
Sodium nitrate on heating evolves nitrogen dioxide
Sodium hydroxide does not decompose on heating
Sodium bicarbonate on heating evolve carbon dioxide
The correct Answer is:C, D
To solve the question of identifying the correct statements regarding the behavior of certain sodium compounds upon heating, we will analyze each option one by one.
1. Option 1: Sodium Carbonate on Heating Evolves Carbon Dioxide
- Sodium carbonate (Na2CO3) does not evolve carbon dioxide upon heating. Instead, it loses its water of crystallization when heated, forming anhydrous sodium carbonate (Na2CO3). Therefore, this statement is false.
2. Option 2: Sodium Nitrate on Heating Evolves Nitrogen Dioxide
- When sodium nitrate (NaNO3) is heated, it decomposes to form sodium nitrite (NaNO2) and oxygen gas (O2), not nitrogen dioxide (NO2). Thus, this statement is also false.
3. Option 3: Sodium Hydroxide Does Not Decompose on Heating
- Sodium hydroxide (NaOH) is thermally stable and does not decompose upon heating. Therefore, this statement is true.
4. Option 4: Sodium Bicarbonate on Heating Evolves Carbon Dioxide
- Sodium bicarbonate (NaHCO3) decomposes upon heating to produce sodium carbonate (Na2CO3), water (H2O), and carbon dioxide (CO2). Thus, this statement is true.
Conclusion:
From the analysis, we find that:
- Option 1: False
- Option 2: False
- Option 3: True
- Option 4: True
The correct statements are Option 3 and Option 4.
- Sodium carbonate (Na2CO3) does not evolve carbon dioxide upon heating. Instead, it loses its water of crystallization when heated, forming anhydrous sodium carbonate (Na2CO3). Therefore, this statement is false.
- When sodium nitrate (NaNO3) is heated, it decomposes to form sodium nitrite (NaNO2) and oxygen gas (O2), not nitrogen dioxide (NO2). Thus, this statement is also false.
- Sodium hydroxide (NaOH) is thermally stable and does not decompose upon heating. Therefore, this statement is true.
- Sodium bicarbonate (NaHCO3) decomposes upon heating to produce sodium carbonate (Na2CO3), water (H2O), and carbon dioxide (CO2). Thus, this statement is true.
From the analysis, we find that:
- Option 1: False
- Option 2: False
- Option 3: True
- Option 4: True
Topper's Solved these Questions
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:MATCH THE COLUMN
Explore 6 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 33 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:Level 3
Explore 47 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWAL ENGLISHChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 3 Videos
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:MATCH THE COLUMN
Explore 3 Videos
Similar Questions
Identify th correct statement(s):
View SolutionThe figure shows a schematic diagram showing the arrangement of Youngs's Double Slit Experiment
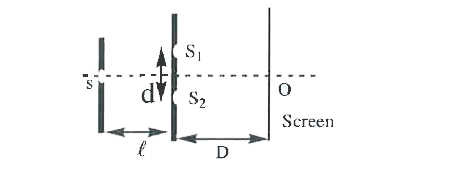
Identify the correct statement(s) if the source slit S moved closer to S1S2, i.e., the distance l decreases
View SolutionThe figure shows a schematic diagram showing the arrangement of Young.s Double Slit Experiment
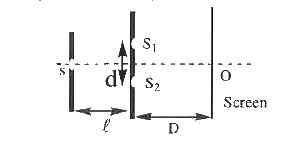
Identify the correct statement (s) if the source slit S moved closer to S1S2 i.e the distance l decreases
View SolutionIdentify the correct statements
View SolutionIdentify the correct statements:
View SolutionIdentify the correct statements:
View SolutionIdentify the correct statements:
View SolutionConsider the Fig. and make the correct option.
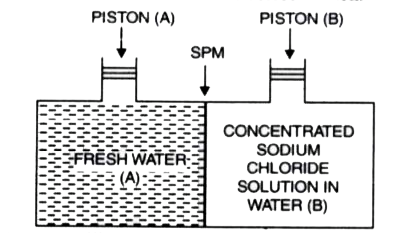
View SolutionIdentify the correct statements
View SolutionThe vapour pressure of two miscible liquids A and B are 300 and 500 mm of Hg respectively.In a flask 2 moles of A are mixed with 6 moles of B.Further to the mixture, 32 g of an ionic non-volatile solute MCl (partically ionised mol.mass =70 u) were also added.Thus, the final vapour pressure of solution was found to be 420 mm of Hg.Then, identify the correct statement(s): (Assume the liquid mixture of A and B to behave ideally ).
View Solution
