Assertion: Be and Mg do not impat characteristic colour to the flame.
Reason: both Be and Mg have high ionization energy.
If both assertion and reason are true and the reason is the correct explanation of assertion
If both assertion and reason are true and the reason is not the correct explanation of assertion
If assertion is false but reason is true
If assertion is true but the reason is false
The correct Answer is:A
Step by step video solution for Assertion: Be and Mg do not impat characteristic colour to the flame. Reason: both Be and Mg have high ionization energy. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - MATCH THE COLUMN
6 Videos
- QUALITATIVE INORGANIC ANALYSISBOOK - VK JAISWAL ENGLISHCHAPTER - QUALITATIVE INORGANIC ANALYSISEXERCISE - SUBJECTIVE PROBLEMS
3 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
Similar Questions
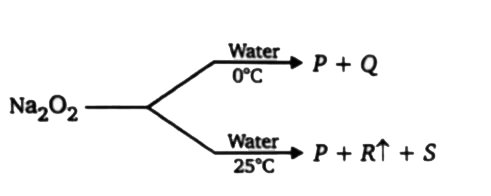
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionEntries of Column-I are to be matched with entrices of Column-II. Each entry of Column-I may have the matching with one or more than entries of Column-II

View SolutionFind number of basic radicals among the following cations, which can form soluble complex on adding excess of NH4 solution.
Cd2+(aq.),Pb2+(aq.),Ni2+(aq.),Mn2+(aq.),Zn2+(aq.),Ag+(aq.),Hg2+(aq.),Fe3+(aq.),Mg2+(aq.)
View SolutionMatch the following columns

View SolutionBe and Mg atoms do not impart colour to the flame.
View SolutionAssertion (A): Be and Mg impart characterstic colour to the flame.
Reason (R ): As compared to other alkaline earth metals, ionisation enthalpy of Be and Mg is low.
View SolutionAssertion : Berylium does not impart any characteristic colour to the Bunsen flame. Reason : Due to its very high ionization energy, beryllium requires a large amount of energy for excitation of the electrons.
View SolutionStatement 1 : Both Be and Mg don’t impart any characteristic colours to the flame
Statement 2 : Both these elements have high value of ionisation enthalpies.
View SolutionAssertion (A): Be and Mg impart characterstic colour to the flame.
Reason (R ): As compared to other alkaline earth metals, ionisation enthalpy of Be and Mg is low.
View SolutionAssertion (A): Alkali metals impart colour to the flame.
Reason (R ): The ionisation energies are low.
View SolutionAssertion: Be and Mg do not impat characteristic colour to the flame. Reason: both Be and Mg have high ionization energy.
View SolutionAssertion : Be and Mg do not impart characteristic colour to the flame.
Reason : Both Be and Mg have high ionisation energy.
View SolutionAssertion: Magnesium does not impact characteristic colour to the bunsen-burner flame.
Reason: Ionisation energy of Mg is very high.
View SolutionAssertion (A) : The first ionization energy of Al is lower than Mg.
Reason (R ) : Atomic radius of Al is smaller than Mg.
View SolutionAssertion: Magnesium does not impact characteristic colour to the bunsen-burner flame.
Reason: Ionisation energy of Mg is very high.
View SolutionAssertion : Be gives characteristic flame colouration.
Reason : Ionization energy of Be is high.
View Solution

