Sodium salt solution +AgNO3soln.→Coloured precipitate.
If coloured precipitate is soluble in both dil. HNO3 and excess conc. NH3 solution then which of the following anion is present in the salt solution?
S2−(aq.)
I−(aq.)
PO3−4(aq.)
Br−(aq.)
The correct Answer is:C
Step by step video solution for Sodium salt solution +AgNO_(3)"soln."toColoured precipitate. If coloured precipitate is soluble in both dil. HNO_(3) and excess conc. NH_(3) solution then which of the following anion is present in the salt solution? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:LEVEL 3
Explore 36 Videos
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:ONE OR MORE ANSWERS IS/ARE CORRECT
Explore 40 Videos
- TYPES OF REACTIONSBook:VK JAISWAL ENGLISHChapter:TYPES OF REACTIONSExercise:MATCH THE COLUMN
Explore 3 Videos
- s-BLOCK ELEMENTSBook:VK JAISWAL ENGLISHChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
Similar Questions
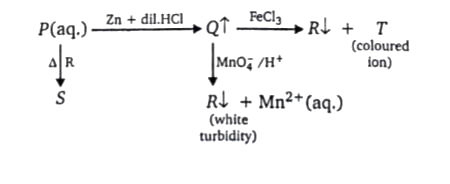
Q. Species P and S are respectively:
View SolutionWhich of the following combination of species can evolve O2?
View SolutionMatch the following columns

View Solution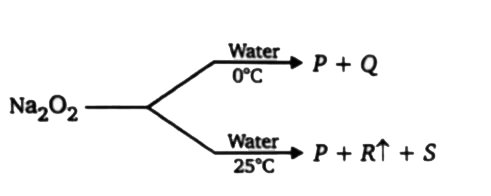
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionA solution of a sodium salt gives yellow precipitate with both Pb^(2+) and Ag^(+) ions. Moreover, the acidified solution of sodium salt with KNO_(2) liberates a coloured gas which turns starch paper blue. The anion is :
View SolutionA white sodium salt dissolves readily in water to give a solution which is neutral to litmus. When silver nitrate solution is added to the solution, a white precipitate is obtained which does not dissolve in dil. HNO_(3) . The anion could be :
View SolutionAqueous solution of a salt when treated with AgNO3 solution gives a white precipitate , white dissolves in NH4OH. Radical present in the salt is
View SolutionIn qualitative analysis when H2S is passed through an aqueous solution of salt acidified with dil. HCl, a black precipitate is obtained. On boiling the precipitate with dil. HNO3, it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives
View SolutionIn qualitative analysis when H2S is passed through an aqueous solution of salt acidified with dil. HCl, a black precipitate is obtained. On boiling the precipitate with dil. HNO3, it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives
View SolutionIn which of the following pairs the precipitates are red and black coloured respectively and both precipitates are soluble in excess Kl solution?
View SolutionSodium salt solution +AgNO3soln.→Coloured precipitate.
If coloured precipitate is soluble in both dil. HNO3 and excess conc. NH3 solution then which of the following anion is present in the salt solution?
View SolutionIn qualitative analysis when H2S is passed through an aqueous solution of salt acidified with dil. HCl, a black precipitate is obtained. On boiling the precipitate with dil. HNO3, it forms a solution of blue colour. Addition of excess of aqueous solution of ammonia to this solution gives
View SolutionWhich of the following precipitate is soluble in excess of NH_(3) solution?
View SolutionSodium salt solution +AgNO3soln.→Coloured precipitate.
If coloured precipitate is soluble in both dil. HNO3 and excess conc. NH3 solution then which of the following anion is present in the salt solution?
View SolutionIn an aqueous solution of an inorganic salt AgNO_3 Is mixed. White precipitate which is obtained NH_4 OH Is soluble in Anion present in salts
View SolutionIn aqueous solution of a salt AgNO_(3) Mixing the solution gives white precipitate which NH_(4)OH Dissolves in The element present in the salt is
View Solution
