Select correct set of species which can't react with water but react with NaOH,
(i) NO2
(ii) P4
(iii) Al
(iv) I2
Only (iv)
(iii) and (iv)
(ii), (iii) and (iv)
all (i), (ii), (iii) and (iv)
The correct Answer is:C
To solve the question of identifying the correct set of species that cannot react with water but can react with NaOH, we will analyze each of the given species one by one.
1. Identify the species:
The species given are:
- (i) NO₂
- (ii) P₄
- (iii) Al
- (iv) I₂
2. Check each species for reaction with water:
- NO₂:
- When NO₂ reacts with water, it forms nitric acid (HNO₃) and nitrous acid (HNO₂).
- Conclusion: NO₂ reacts with water.
- P₄:
- Phosphorus (P₄) does not react with water under normal conditions.
- Conclusion: P₄ does not react with water.
- Al:
- Aluminum (Al) does not react with water directly but can react with steam or hot water to form aluminum oxide and hydrogen gas. However, under normal conditions, it does not react with cold water.
- Conclusion: Al does not react with water under normal conditions.
- I₂:
- Iodine (I₂) does not react with water.
- Conclusion: I₂ does not react with water.
3. Check each species for reaction with NaOH:
- NO₂:
- When NO₂ reacts with NaOH, it forms sodium nitrite (NaNO₂) and sodium nitrate (NaNO₃).
- Conclusion: NO₂ reacts with NaOH.
- P₄:
- When P₄ reacts with NaOH, it can form sodium hypophosphite (NaH₂PO₂) and phosphine (PH₃).
- Conclusion: P₄ reacts with NaOH.
- Al:
- Aluminum reacts with NaOH to form sodium aluminate (NaAlO₂) and hydrogen gas.
- Conclusion: Al reacts with NaOH.
- I₂:
- Iodine reacts with NaOH to form sodium iodide (NaI) and sodium hypoiodite (NaOI).
- Conclusion: I₂ reacts with NaOH.
4. Summarize the findings:
- Species that do not react with water: P₄, Al, I₂
- Species that react with NaOH: P₄, Al, I₂
5. Final answer:
The correct set of species that cannot react with water but can react with NaOH is:
- (ii) P₄
- (iii) Al
- (iv) I₂
The species given are:
- (i) NO₂
- (ii) P₄
- (iii) Al
- (iv) I₂
- NO₂:
- When NO₂ reacts with water, it forms nitric acid (HNO₃) and nitrous acid (HNO₂).
- Conclusion: NO₂ reacts with water.
- P₄:
- Phosphorus (P₄) does not react with water under normal conditions.
- Conclusion: P₄ does not react with water.
- Al:
- Aluminum (Al) does not react with water directly but can react with steam or hot water to form aluminum oxide and hydrogen gas. However, under normal conditions, it does not react with cold water.
- Conclusion: Al does not react with water under normal conditions.
- I₂:
- Iodine (I₂) does not react with water.
- Conclusion: I₂ does not react with water.
- NO₂:
- When NO₂ reacts with NaOH, it forms sodium nitrite (NaNO₂) and sodium nitrate (NaNO₃).
- Conclusion: NO₂ reacts with NaOH.
- P₄:
- When P₄ reacts with NaOH, it can form sodium hypophosphite (NaH₂PO₂) and phosphine (PH₃).
- Conclusion: P₄ reacts with NaOH.
- Al:
- Aluminum reacts with NaOH to form sodium aluminate (NaAlO₂) and hydrogen gas.
- Conclusion: Al reacts with NaOH.
- I₂:
- Iodine reacts with NaOH to form sodium iodide (NaI) and sodium hypoiodite (NaOI).
- Conclusion: I₂ reacts with NaOH.
- Species that do not react with water: P₄, Al, I₂
- Species that react with NaOH: P₄, Al, I₂
The correct set of species that cannot react with water but can react with NaOH is:
- (ii) P₄
- (iii) Al
- (iv) I₂
Topper's Solved these Questions
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - LEVEL 3
36 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - ONE OR MORE ANSWERS IS/ARE CORRECT
40 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
Similar Questions
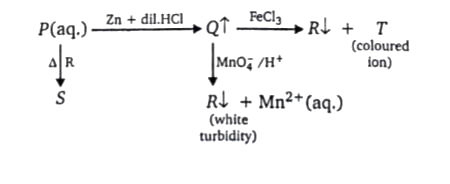
Q. Species P and S are respectively:
View SolutionWhich of the following combination of species can evolve O2?
View SolutionMatch the following columns

View Solution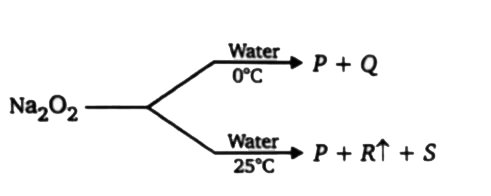
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionThe oxides which cannot act as reductant:
(I) CO2 (II) SO3 (III) P4O10 (IV) NO2
View SolutionAcetone reacts with I2 in presence of NaOH to from
View SolutionGlycerol reacts with P_(4) + I_(2) to form
View SolutionBe and Al exhibit diagonal relationship . Which of the following statements about them is/are not true ?
(i) Both react with HCl to liberate H2.
(ii) They are made passive by HNO3.
(iii) Their carbides give acetylene on treatment with water .
(iv) Their oxides are amphoteric .
View SolutionCorrect order for N-O bond length in the given species is underset(I)(NO) underset(II) (NO^(+)) underset(III)(NO_(2)^(-)) underset(IV)(NO_(3)^(-))
View Solutionनिम्नलिखित यौगिकों की जल से क्रिया करने पर प्राप्त विलयन अम्लीय होगा अथवा क्षारीय-
(i)Na2O (ii) SO2 (iii) P4O6
View Solutionक्या होता है जब-
(i) बेरिलियम क्लोराइड NaOH से अभिकृत किया जाता है ?
(ii) कैल्सियम हाइड्राइड को जल से अभिकृत किया जाता है ?
(iii) कार्बन डाइऑक्साइड को चुने के पानी में प्रवाहित किया जाता है ?
View SolutionSelect correct set of species which can't react with water burt react with NaOH,
(i) NO2
(ii) P4
(iii) Al
(iv) I2
View SolutionEthanol & propanone react with I_(2)//NaOH to form _______
View SolutionAcetone reacts with I_(2) in presence of NaOH to form:
View SolutionGlycerol reacts with P_(4)+I_(2) to form:
View Solution

