X2Sn+water→X(OH)n⏐⏐
⏐
⏐↓+Y↑(Gas)Pb(CH3COO)2−−−−−−−−−→Z↓(Black ppt.)
Then (X) cation can not be:
Fe3+ gives brown colour solution with potassium ferricyanide
Al3+
Cr3+
Mg2+
The correct Answer is:A
Step by step video solution for X_(2)S_(n)+water to X(OH)_(n) darr+ underset((Gas))(Y uarr)overset(Pb(CH_(3)COO)_(2))tounderset(("Black ppt."))(Z darr) Then (X) cation can not be: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - LEVEL 3
36 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - ONE OR MORE ANSWERS IS/ARE CORRECT
40 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
Similar Questions
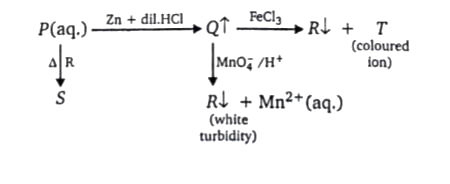
Q. Species P and S are respectively:
View SolutionWhich of the following combination of species can evolve O2?
View SolutionMatch the following columns

View Solution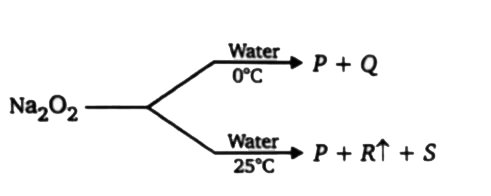
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionX(Black)Dil.H2SO4−−−−−−→Y(gas)Dil.HNO3−−−−−−→ Colloidal sulphur identify X?
View Solution(X)Ba2+−−→Y(white ppt)⏐⏐ ⏐ ⏐↓Cl2/H2O−−−−−→ZWhite ppt⏐⏐ ⏐ ⏐↓
If the precipitate (Z⏐↓) is BaSO4, which of the following can be (X)?
View SolutionIn the following sequence in aqueous solution, the species X, Y and Z, respectively, are S_(2)O_(3)^(2-) overset(Ag^(+))rarrunderset(("Clear solution"))X overset (Ag^(+))rarr underset(("White ppt."))Y overset("With time")rarr underset(("Black ppt."))Z
View SolutionThe compounds X and Y in the following reaction are
CH3−CHCl2Zn dust−−−−→CH3OH↓Ethonic KOHY X
View SolutionIn the following reaction which bonds are cleaved? CH_(3)CH_(2)-overset(O)overset(||)underset("I")Ooverset(q)overset(darr)-Ooverset(p)overset(darr)-H+CH_(3)CH_(2)overset(s)overset(darr)underset("II")-Ooverset(r)overset(darr)-H
View SolutionA compound X declourises Br2 water and reacts slowly with conc H2SO4 to give an addition product. X reacts with HBr to form Y. Y reacts with NaOH to form Z. on oxidation Z gives hexan-3-one. X, Y and Z in the reactions are
X↓conc. H2SO4Addition productBr2 water−−−−−→Decolourisation
XHBr−−→YNaOH−−−→Z[O]−−→CH3CH2CH2−C∣∣O−CH2CH3Hexan-3-one
View SolutionX_(2)S_(n)+water to X(OH)_(n) darr+ underset((Gas))(Y uarr)overset(Pb(CH_(3)COO)_(2))tounderset(("Black ppt."))(Z darr) Then (X) cation can not be:
View SolutionMn+(aq.)+KI→X↓ppt.Excess−−−→KI ppt. remains insoluble in excess KI solution. Then cation Mn+(aq.) can be:
View SolutionHypothetical scheme is given below for one molecule of acids.
H2O2SO3−−→H2SO5SO3−−→(X)↓Peroxomonosulphuric acidH2O2SO2−−→H2S2O3SO3−−→(Y)↓Thiosulphuric acidH2OnSO3−−→H2S(n+1)O3SO3−−→(Z)
Find the sum of peroxylinkage in X,Y and Z.
View SolutionMn+(aq.)+KI→X↓ppt.Excess−−−→KI ppt. remains insoluble in excess KI solution. Then cation Mn+(aq.) can be:
View SolutionMSO4NH4OH−−−−→⏐⏐ ⏐ ⏐↓XसफेदNH4OH−−−−→आधिक्यYH2S−−→⏐⏐ ⏐ ⏐↓Z यहाँ M और Z है
View SolutionCH3−CH3↑C↓CH3−NCreduction−−−−−→?
View Solution

