A monochromatic beam of light having photon energy 12.5eV is incident on a simple A of atomic hydrogen gas in which all almost are in the ground state. The emission spectra obtained from this sample is incident on another sample B of atomic hydrogen gas in which all atoms are in the first excited state.
Based on above information, answer the following question:
The emission spectra of sample A
must have 3 lines
must have 2 lines
may have 2 lines
it is not formed
The correct Answer is:D
To solve the problem, we need to analyze the situation involving the emission spectra of atomic hydrogen when exposed to a monochromatic beam of light with a photon energy of 12.5 eV.
1. Understanding the Energy Levels of Hydrogen:
- The energy levels of hydrogen are given by the formula:
En=−13.6eVn2
- For the ground state (n=1):
E1=−13.6eV
- For the first excited state (n=2):
E2=−3.4eV
- For the second excited state (n=3):
E3=−1.51eV
- For the third excited state (n=4):
E4=−0.85eV
2. Photon Energy Incident on Sample A:
- The incident photon energy is given as 12.5 eV. This energy is much higher than the energy required to excite electrons from the ground state to any of the higher energy levels.
3. Calculating the Energy Gap:
- The energy required to excite an electron from the ground state (n=1) to the first excited state (n=2) is:
E2−E1=−3.4eV−(−13.6eV)=10.2eV
- The energy required to excite an electron from the ground state to the second excited state (n=3) is:
E3−E1=−1.51eV−(−13.6eV)=12.09eV
- The energy required to excite an electron from the ground state to the third excited state (n=4) is:
E4−E1=−0.85eV−(−13.6eV)=12.75eV
4. Determining Possible Transitions:
- The photon energy of 12.5 eV can excite electrons to the second excited state (n=3) since it is less than 12.75 eV but greater than 12.09 eV.
- However, it cannot excite electrons to the first excited state (n=2) since the required energy is 10.2 eV, and it can also excite to n=3.
5. Emission Spectra from Sample A:
- Since the electrons can transition from the second excited state (n=3) back to the ground state (n=1), we can expect emission lines corresponding to these transitions.
- The possible transitions from n=3 to n=1 will produce one emission line.
6. Conclusion:
- As the only transition possible is from n=3 to n=1, the emission spectra of sample A will consist of one line.
Final Answer:
The emission spectra of sample A will have one line.
- The energy levels of hydrogen are given by the formula:
En=−13.6eVn2
- For the ground state (n=1):
E1=−13.6eV
- For the first excited state (n=2):
E2=−3.4eV
- For the second excited state (n=3):
E3=−1.51eV
- For the third excited state (n=4):
E4=−0.85eV
- The incident photon energy is given as 12.5 eV. This energy is much higher than the energy required to excite electrons from the ground state to any of the higher energy levels.
- The energy required to excite an electron from the ground state (n=1) to the first excited state (n=2) is:
E2−E1=−3.4eV−(−13.6eV)=10.2eV
- The energy required to excite an electron from the ground state to the second excited state (n=3) is:
E3−E1=−1.51eV−(−13.6eV)=12.09eV
- The energy required to excite an electron from the ground state to the third excited state (n=4) is:
E4−E1=−0.85eV−(−13.6eV)=12.75eV
- The photon energy of 12.5 eV can excite electrons to the second excited state (n=3) since it is less than 12.75 eV but greater than 12.09 eV.
- However, it cannot excite electrons to the first excited state (n=2) since the required energy is 10.2 eV, and it can also excite to n=3.
- Since the electrons can transition from the second excited state (n=3) back to the ground state (n=1), we can expect emission lines corresponding to these transitions.
- The possible transitions from n=3 to n=1 will produce one emission line.
- As the only transition possible is from n=3 to n=1, the emission spectra of sample A will consist of one line.
The emission spectra of sample A will have one line.
Topper's Solved these Questions
- ATOMIC PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - ATOMIC PHYSICSEXERCISE - Integer
4 Videos
- ATOMIC PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - ATOMIC PHYSICSEXERCISE - Fill In The Blanks
8 Videos
- ATOMIC PHYSICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - ATOMIC PHYSICSEXERCISE - Multiple Correct
13 Videos
- ALTERNATING CURRENTBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - ALTERNATING CURRENTEXERCISE - Single Correct
10 Videos
- CAPACITOR AND CAPACITANCEBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - CAPACITOR AND CAPACITANCEEXERCISE - Integer
5 Videos
Similar Questions
In the spectrum of singly ionized helium , the wavelength of a line abserved is almost the same as the first line of Balmer series of hydrogen . It is due to transition of electron from n1=6→n2=''∗''. What is the value of (''∗'').
View SolutionTo produce characteristic X - rays using a Tungsten target in an X - ray generator , the accelerating should be greater than ……. Volts and the energy of the characterization is …… eV .
(The binding energy of the intermost electron in Tungsten is - 40keV).
View SolutionWhich of the following statements are true?
View SolutionThe rms current in an AC circuit is 2A. If the wattless current be √3A , what is the power factor of the circuit?
View SolutionEach capacitance shown in is in μF. Find the charge on 6μF.
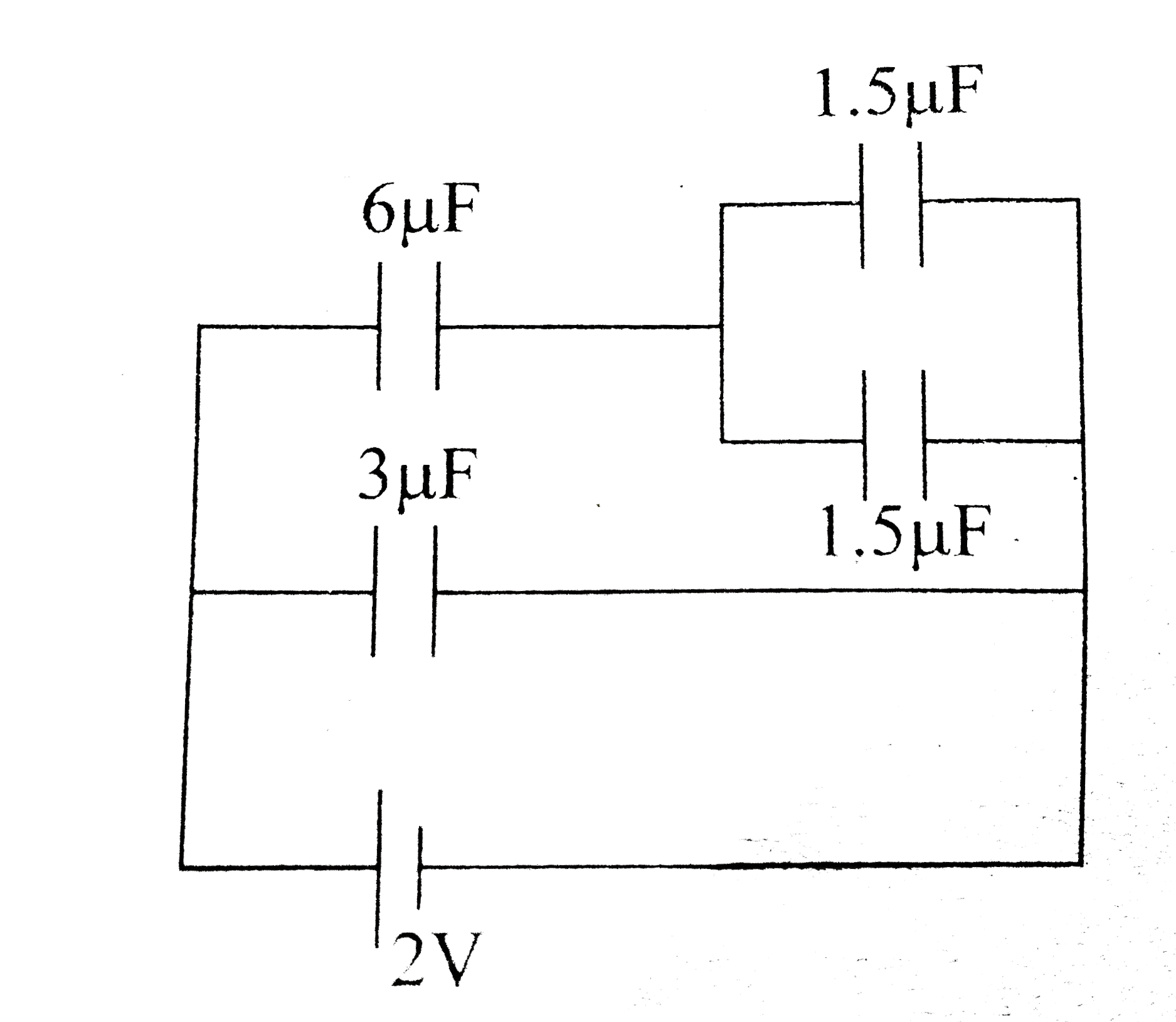 .
.
View SolutionWhich wavelength will be emitting by a sample of atomic hydrogen gas (in ground state) if electron of energy 12.5eV collide with the atoms of the gas?
View SolutionHydrogen aton in a sample are excited to n = 5 state and it is found that photons of all possible wavelength are present in the emission spectra. The min imum number of hydrogen atom in the sample would be
View SolutionA monochromatic beam of light having photon energy 12.5 eV is incident on a simple A of atomic hydrogen gas in which all almost are in the ground state. The emission spectra obtained from this sample is incident on another sample B of atomic hydrogen gas in which all atoms are in the first excited state. Based on above information, answer the following question: The atoms of sample A after passing of light through it
View SolutionA monochromatic beam of light having photon energy 12.5 eV is incident on a simple A of atomic hydrogen gas in which all almost are in the ground state. The emission spectra obtained from this sample is incident on another sample B of atomic hydrogen gas in which all atoms are in the first excited state. Based on above information, answer the following question: The emission spectra of sample A
View SolutionA monochromatic beam of light having photon energy 12.5eV is incident on a simple A of atomic hydrogen gas in which all almost are in the ground state. The emission spectra obtained from this sample is incident on another sample B of atomic hydrogen gas in which all atoms are in the first excited state.
Based on above information, answer the following question:
The atoms of sample B
View SolutionHydrogen atoms in the ground state are excited by monochromatic radiation photons of energy 12.1 eV. To which orbit the electron will be lifted, if an hydrogen atom absorbs the photon ?
View SolutionElectrons having kinetic energy 30 eV are made to collide with atomic hydrogen gas (in ground state) and 42.5% of electron energy is used to excite the hydrogen wavelength in emission spectra are
View SolutionA: If a beam of photons of energy 10.0 eV each is incident on a sample of hydrogen gas containing all atoms in the ground state, then the beam of the photons is completely transmitted through the gas without absorption.
R: The minimum energy required by an electron to make a transition to an excited state is 10.2eV.
View SolutionHydrogen atom is excited from ground state to another state with principal quantum number equal to 5. Then the number of spectral lines in the emission spectra will be:
View SolutionA photon beam of energy 12.1 eV is incident on a hydrogen atom. The orbit to which electron of H-atom be excited is
View Solution

