Two moles of helium gas are taken along the path ABCD (as shown in Fig.) The work done by the gas is

2000R(12+1n43)
500R(R+1n4)
500R(2+1n169)
1000R(1+1n169)
The correct Answer is:A
a. A→B is isobaric process,
V∝T
so, ΔWAB=nRΔT=2×R×(750−250)=1000R
B→C is an isochoric process
∴ΔWBC=0 and
C→D is an isothermal process
ΔWCD=nRT1n(VfVi)
=2×R×10001n(2015)=20001n(43)
Total work done, ΔW=ΔWAB+ΔWBC+ΔWCD
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Multiple Corrects
28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Subjective
22 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
Similar Questions
Two moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
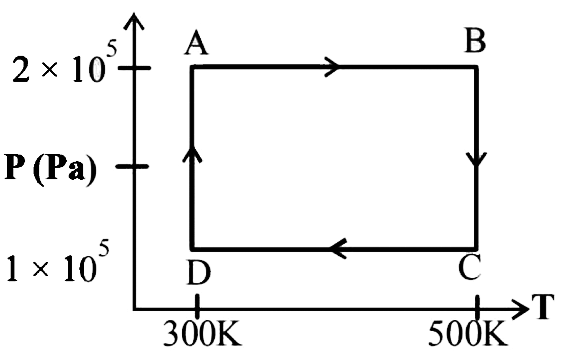
The work done on the gas in taking it from D to A is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

The work done on the gas in taking it from D to A is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
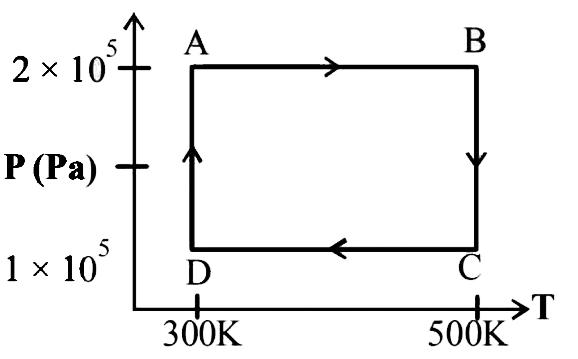
The net work done on the gas in the cycle ABCDA is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

The net work done on the gas in the cycle ABCDA is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
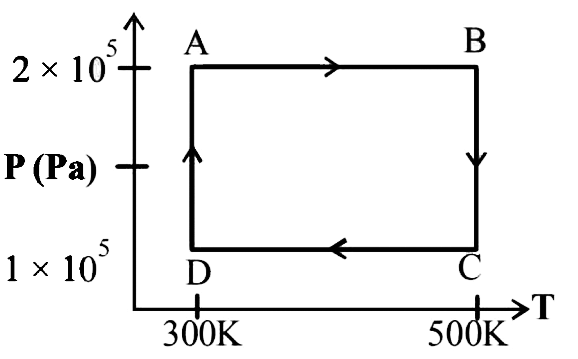
Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram

Assuming the gas to be ideal the work done on the gas in taking it form A to B is :
View SolutionAn ideal gas is taken around the cycle ABCA as shown in P-V diagram. The net work done by the gas during the cycle is equal to :
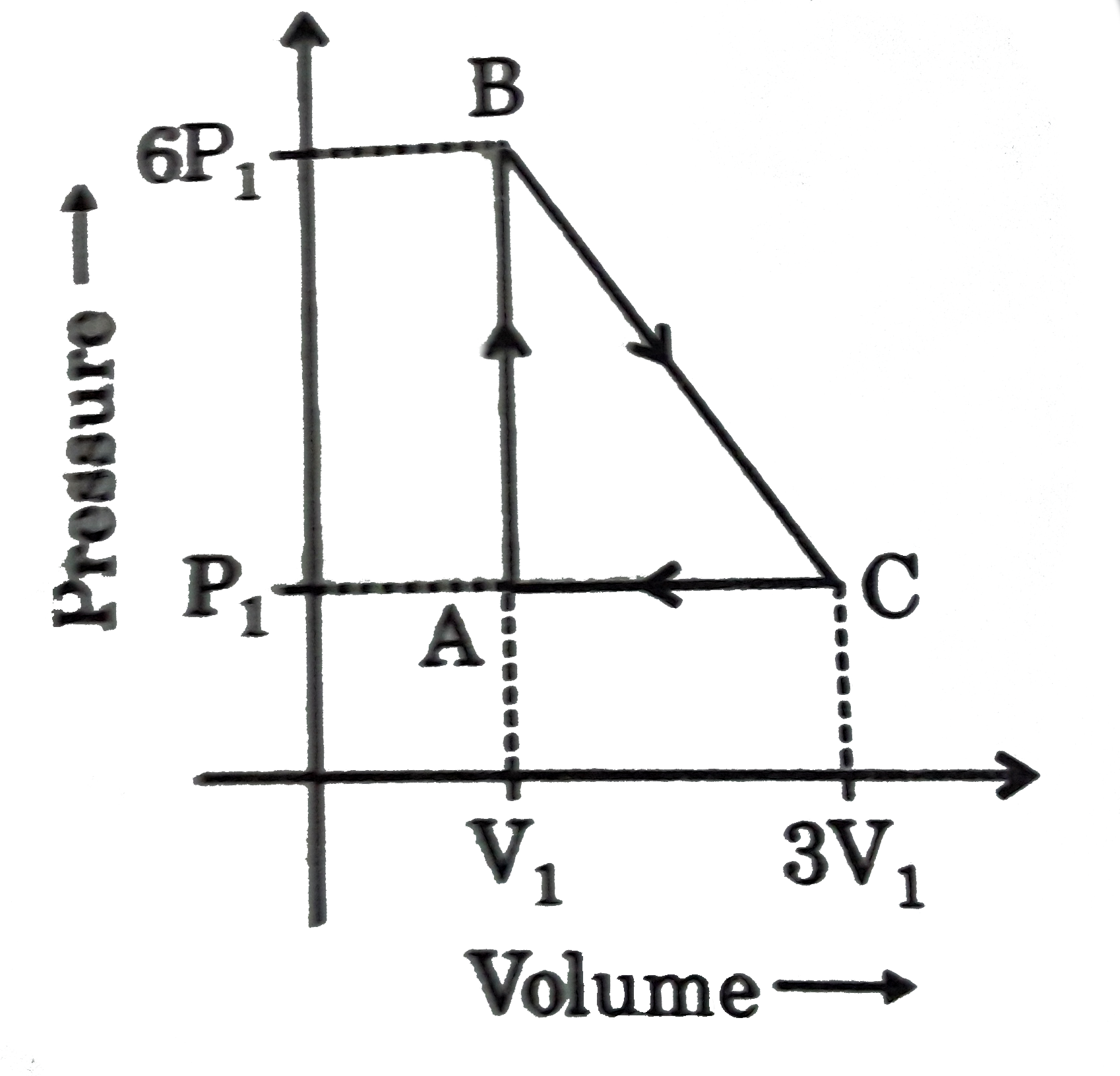
View SolutionAn ideal gas is taken around the cycle ABCA as shown in P-V diagram. The net work done by the gas during the cycle is equal to :

View SolutionVolume versus temperature graph of two moles of helium gas is as shown in figure. The ratio of heat absorbed and the work done by the gas in process 1−2 is
View SolutionVolume versus temperature graph of two moles of helium gas is as shown in figure. The ratio of heat absorbed and the work done by the gas in process 1−2 is

View Solution

