One mole of an ideal monatomic gas has initial temperature T0, is made to go through the cycle abca shown in Fig. If U denotes the internal energy, then choose the correct alternative
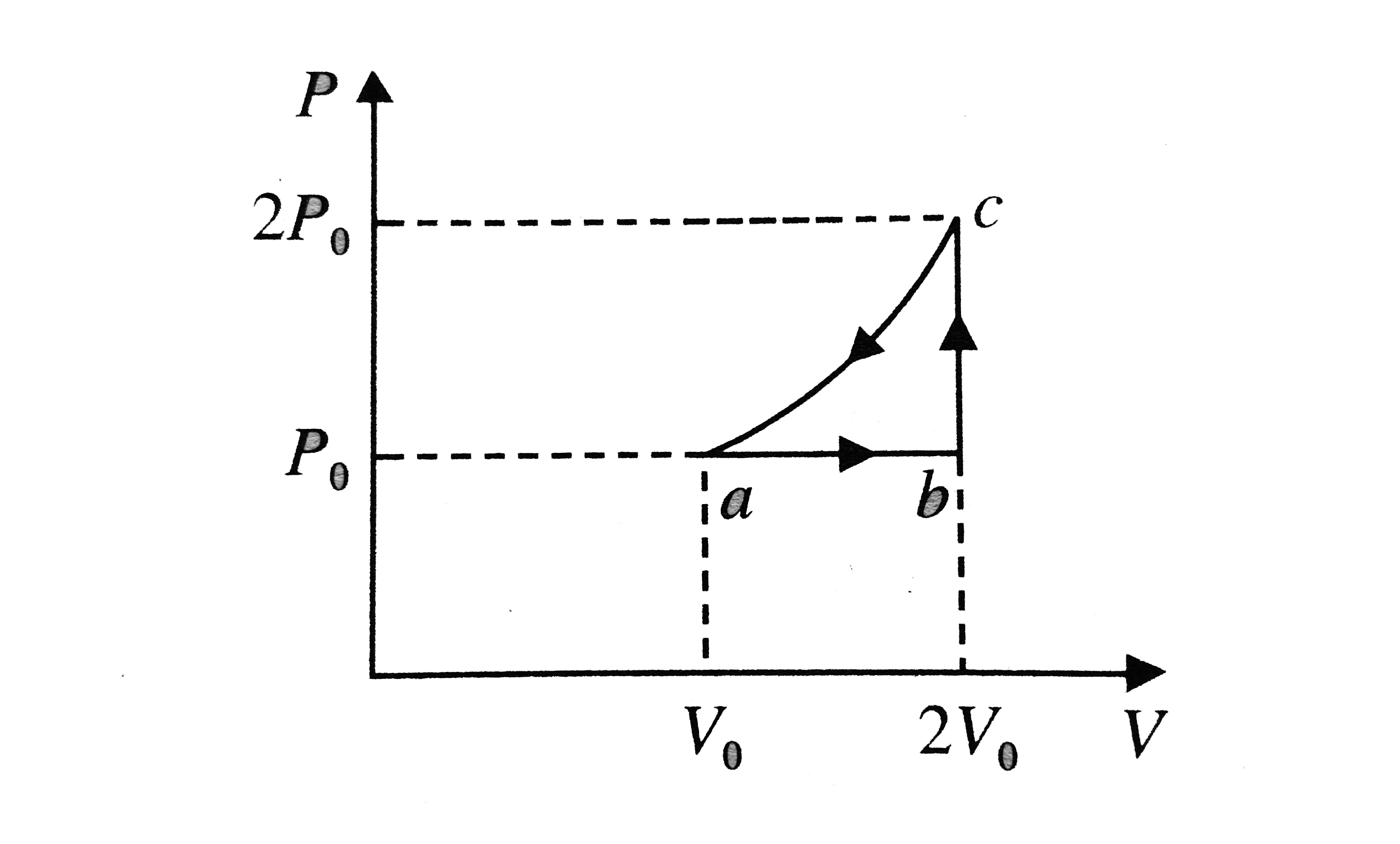
UC>Ub>Ua
UC−Ub=3RT0
Uc−Ua=9RT02
Ub−Ua=3RT02
The correct Answer is:A, B, C, D
One mole of an ideal monatomic gas ( initial temperature T0) is made to go through cylce abca shown in figure. U denotes the internal energy.
For the process ab,P0V0T0=2P0V0T0
Tb=2T0
Tb>Ta⇒Ub>Ua
Ub−Ua=C,ΔT=3R2(2T0−T0)=3RT02
For the process bc,
Ub−Ua=3R2(4T0−2T0)=3RT0
For the process ca, Uc−Ua=3R2(4T0−T0)=9RT2
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Comprehension
56 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Single Correct
140 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
Similar Questions
One mole of an ideal monatomic gas (intial temperature T0) is made to go through the cycle abca as shown in the figure. If U denotes the internal energy, then choose the correct alternatives
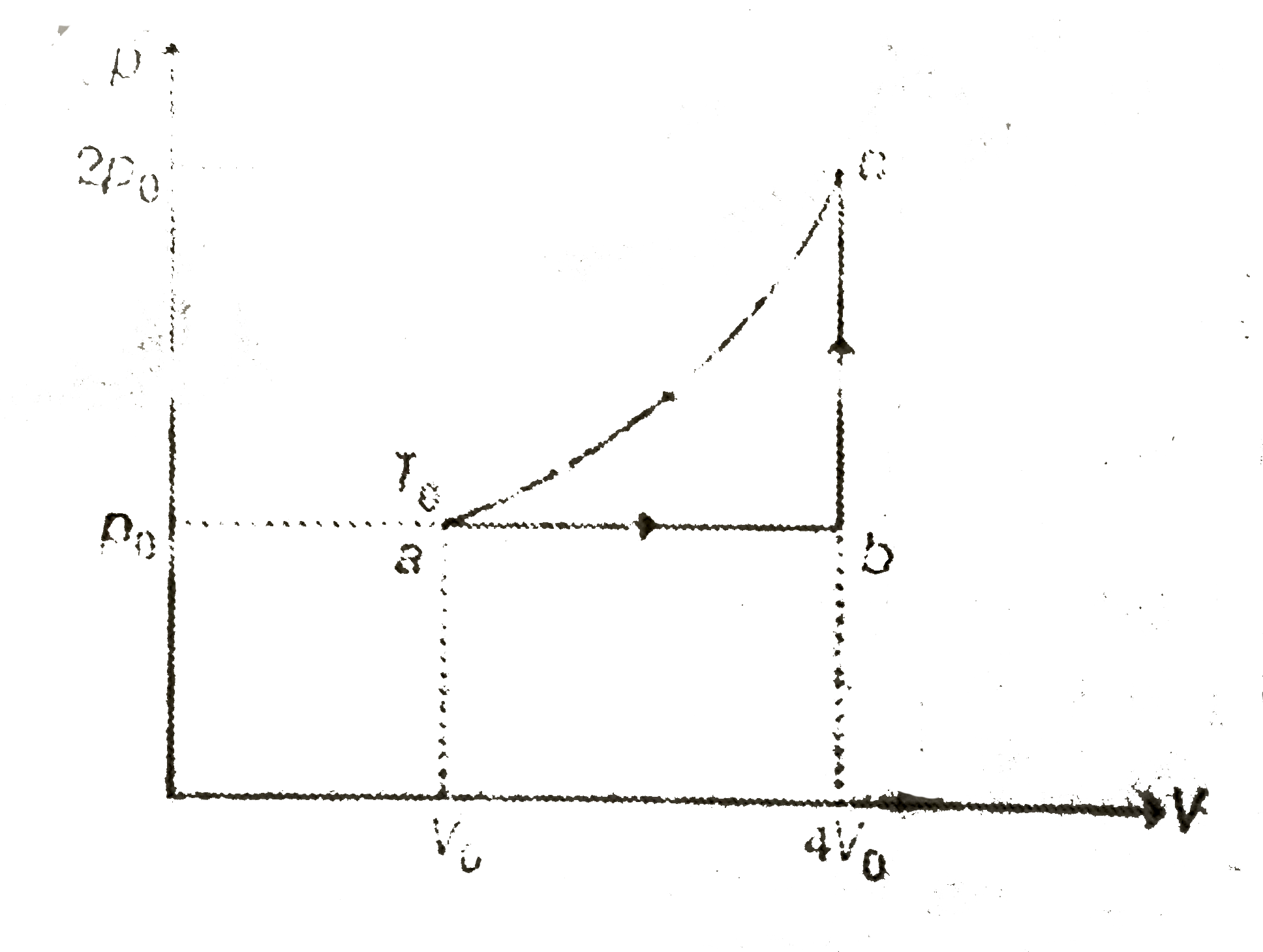
View SolutionOne mole of an ideal monoatomic gas is caused to go through the cycle shown in figure. Then the change in the internal energy of gas from a to b and b to c is respectively
View Solution
Knowledge Check
One mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
A2RT0BRToC32RT0D12RT0One mole of an ideal monatomic gas at temperature T0 expands slowly according to the law PV=constant.If the final temperature is 2T0, heat supplied to the gas is
A2RT0BRT0C32RT0D12RT0One mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
Aa.2RT0Bb.RT0Cc.32RT0Dd.12RT0
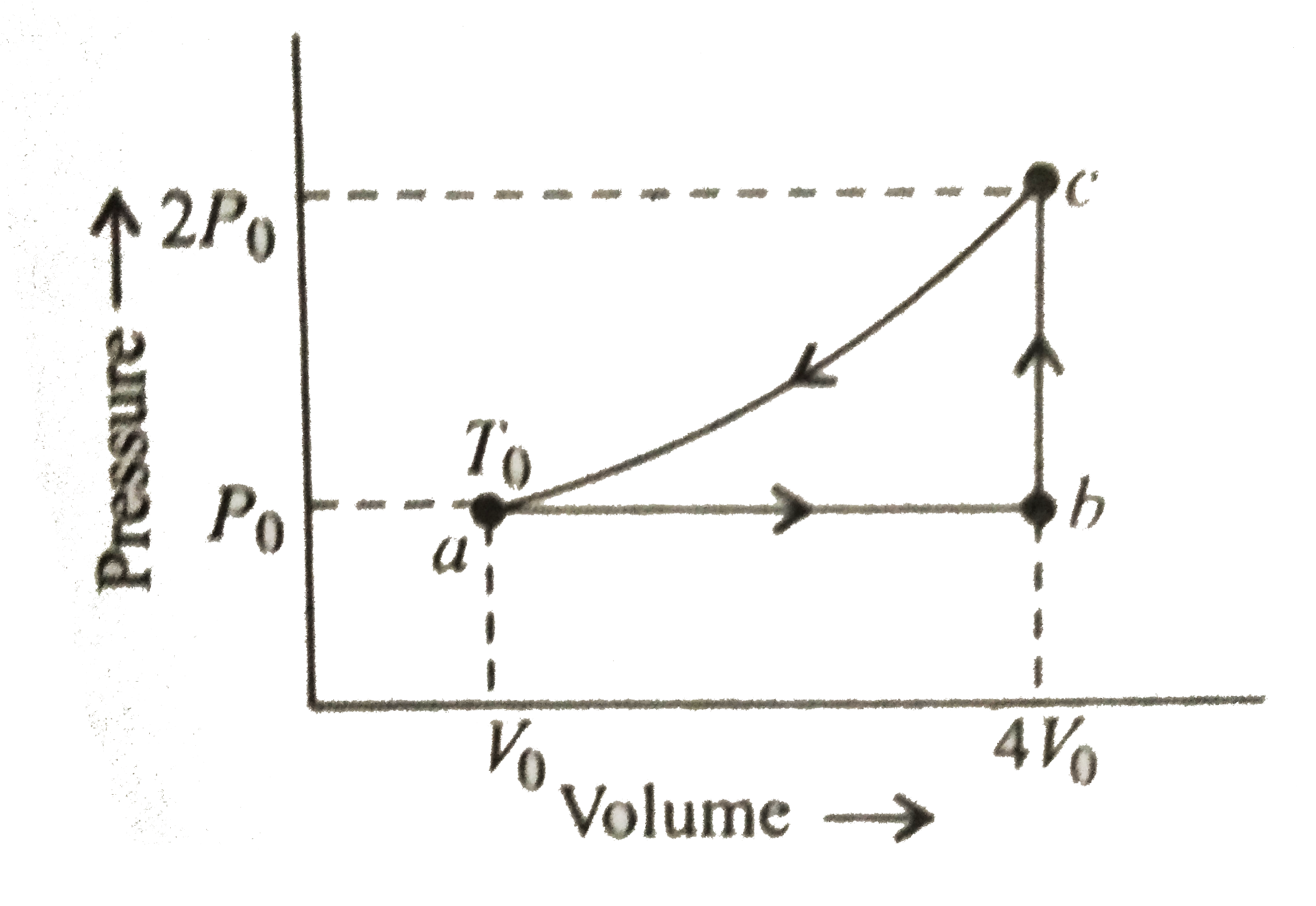
One mole of an ideal mono-atomic gas is caused to go through the cycle shown in the figure below. Then the change in the internal enegry in expanding the gas from a to c along the path abc is:
View SolutionOne mole of an ideal monoatomic gas is taken at a temperature of 300K. Its volume is doubled keeping its pressure constant. Find the change in internal energy.
View SolutionOne mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law pV = constant. If the final temperature is 2T0, heat supplied to the gas is
View SolutionOne mole of an ideal monatomic gas at temperature T0 expands slowly according to the law P=kV (k is constant). If the final temperature is 4T0 then heat supplied to gas is
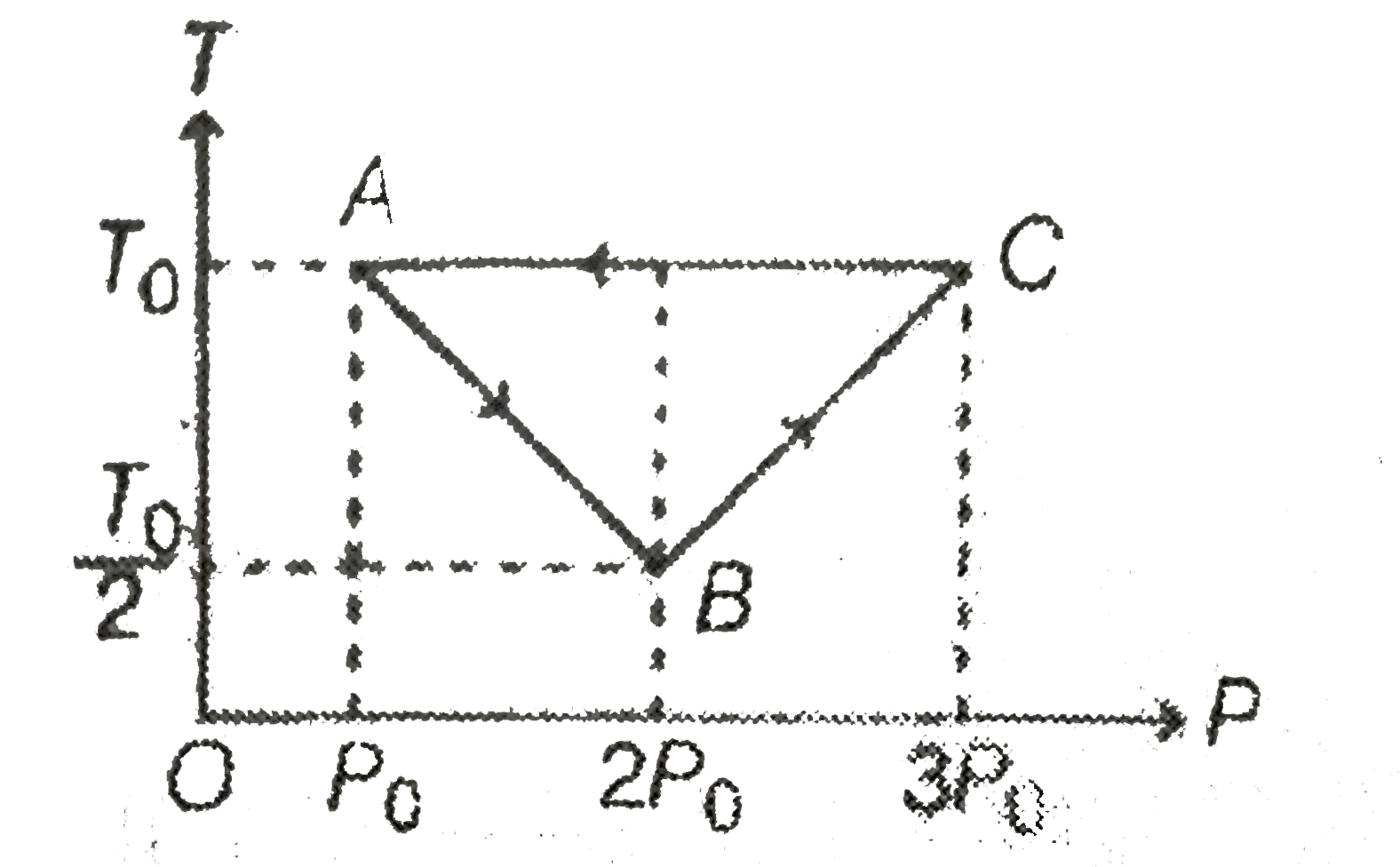
View SolutionOne mole of an ideal monoatomic gas is taken through a cyclic process as shown. Choose the correct option(s).
View Solution

