Piston cylinder device initially contains 0.5m3 of nitrogen gas at 400kPa and 27∘C. An electric heater within the device is turned on and is allowed to apss a current of 2A for 5min from a 120V source. Nitrogen expands at constant pressure and a heat loss of 2800J occurs during the process.
R=25/3kJ/kmol−K
Number of moles of nitrogen gas is
2.6∘C
56.6∘C
29.6∘C
5.67∘C
The correct Answer is:B
Heat loss, Q1=2800J
Work done at constant pressure =nCpΔT
(∵Cp=7R2=72×253=1756kJ/mol−K)
Q−WC=nCpΔT
−2.8kJ−(−72kJ)=0.08×1756ΔT
⇒ΔT=(69.2kJ)(0.08kmol)(1756−kJ/kmol−K)
=29.6K
Final temperature =27+29.6
=56.6∘C
Topper's Solved these Questions
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Interger
11 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
Similar Questions
8g of oxygen, 14 g of nitrogen and 22 g carbon dioxide are mixed in an encloser of volume 10 litre and temperature 27∘C. Calculate the pressure exerted by the mixture , R=8.3Jmole−1K−1, Molecular weight of oxygen , nitrogen and carbon 32 , 28 and 44 respectively.
View SolutionStatement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
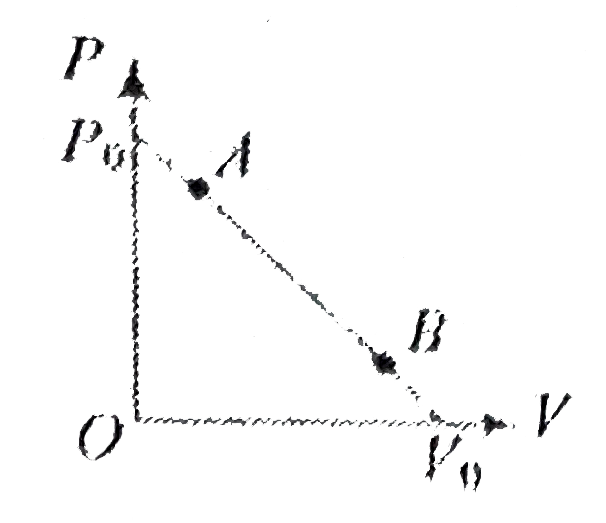
The absolute temperature T versus volume V for the given process is
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
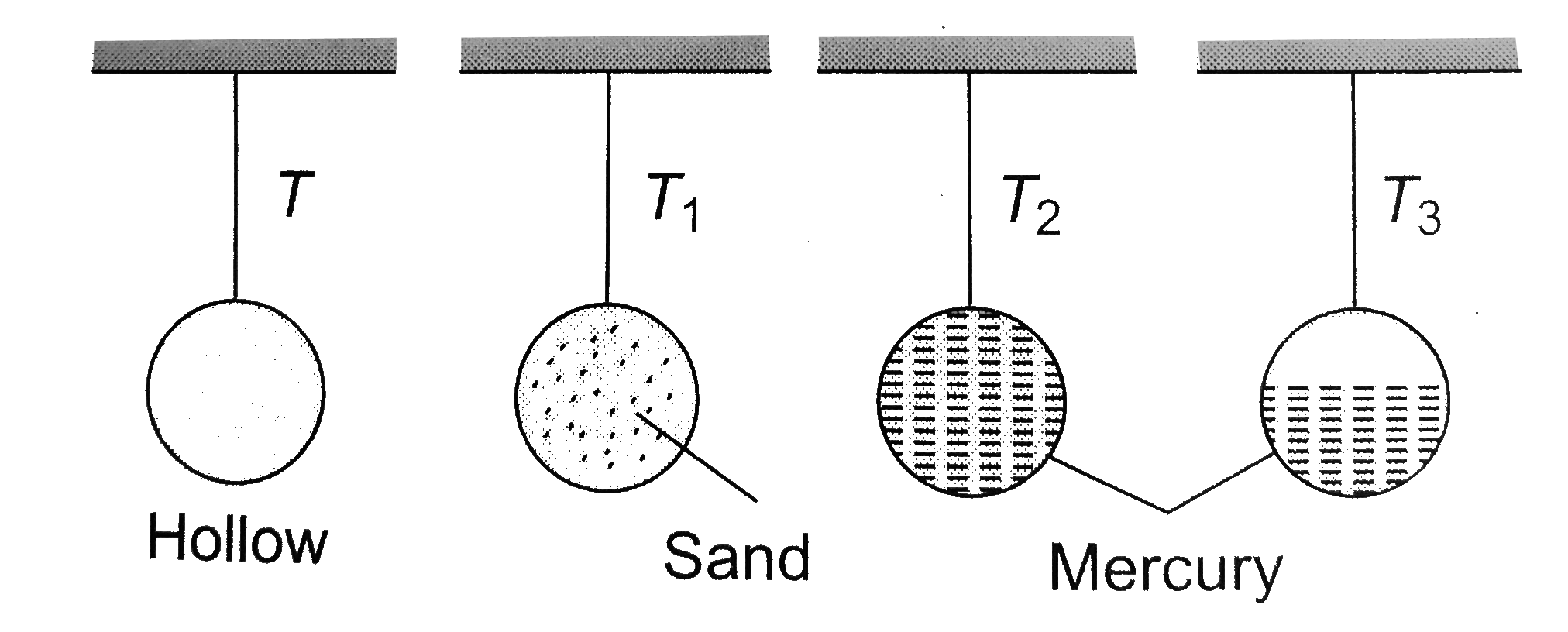
View SolutionPiston cylinder device initially contains 0.5m^(3) of nitrogen gas at 400 kPa and 27^(@)C . An electric heater within the device is turned on and is allowed to apss a current of 2 A for 5 mi n from a 120 V source. Nitrogen expands at constant pressure and a heat loss of 2800 J occurs during the process. R=25//3 kJ //k mol - K Electric work done on the nitrogen gas is
View SolutionPiston cylinder device initially contains 0.5m^(3) of nitrogen gas at 400 kPa and 27^(@)C . An electric heater within the device is turned on and is allowed to apss a current of 2 A for 5 mi n from a 120 V source. Nitrogen expands at constant pressure and a heat loss of 2800 J occurs during the process. R=25//3 kJ //k mol - K Number of moles of nitrogen gas is
View SolutionPiston cylinder device initially contains 0.5m3 of nitrogen gas at 400kPa and 27∘C. An electric heater within the device is turned on and is allowed to pass a current of 2A for 5min from a 120V source. Nitrogen expands at constant pressure and a heat loss of 2800J occurs during the process.
R=25/3kJ/kmol−K
The final temperature of nitrogen is
View SolutionTwo moles of nitrogen gas is kept in a cylinder of cross-section area 10cm3. The cylinder is closed by a light frictionless piston. Now the gas is slowly heated such that the displacement of piston during process is 50cm, find the rise in temperature of gas when 200J of heat is added in it. (Atmospheric pressure =100kPa,R=25/3J/mol−K)
View Solution1 mole of an ideal gas expands from 5dm3 to25dm3 isothermally and irreversibly at 27∘ C. Find work done in the process [R=8.3 J/mol/K]
View SolutionOne mole of an ideal gas is allowed to expand reversible and adiabatically from a temperatureof27∘C)if the work done during the process is3kJ,the final temperature will be equal to(Cv=20JK−1)
View SolutionOne mole of an ideal gas is allowed to expand reversible and adiabatically from a temperatureof27∘C)if the work done during the process is3kJ,the final temperature will be equal to(Cv=20JK−1)
View Solution0.5 मोल नाइट्रोजन को स्थिर आयतन पर 50∘C से 70∘C तक गर्म किया जाता है। गेस की आन्तरिक ऊर्जा में परिवर्तन की गणना कीजिए। नाइट्रोजन की स्थिर दाब पर विशिष्ट ऊष्मा Cp=7 कैलोरी/मोल-K तथा सार्वत्रिक गैस नियतांक R=2 कैलोरी/मोल −K
View SolutionA cylinder contains hydrogen gas at pressure of 249 kPa and temperature 27°C. It's density is
(Given,R=8.3Jmol−1K−1)
View SolutionThe nitrogen gas pressure inside a container of volume 2.6cm3 is 2.3 atm at 27∘C . The approximate number of moles of nitrogen present in the container is
[ R=0.0821 L atm mol1K−1 ]
View SolutionTwo moles of an ideal monatomic gas are confined within a cylinder by a massless and frictionless spring loaded piston of cross-sectional area 4 xx 10^(-3) m^2 . The spring is, initially, in its relaxed state. Now the gas is heated by an electric heater, placed inside the cylinder, for some time. During this time, the gas expands and does 50 J of work in moving the piston through a distance 0.10 m. The temperature of the gas increases by 50 K. Calculate the spring constant and the heat supplied by the heater.
View SolutionAt 27∘C two moles of an ideal monatomic gas occupies a volume V. The gas expands adiabatically to a volume 2V. Calculate
the work done by the gas during the process. (R = 8.31 J/mol K)
View Solution

