The shape of orbitals are related to the ratio of principal quantum number (n) to substiary quantum number (k,a modifacation of Bohr-sommerfield theory ).The value of k for any shell has a value ranging betwe3en n to l .The amximum value for k is given for x sub-shell white k becomes with p, d,f........ repectively upto minimum value
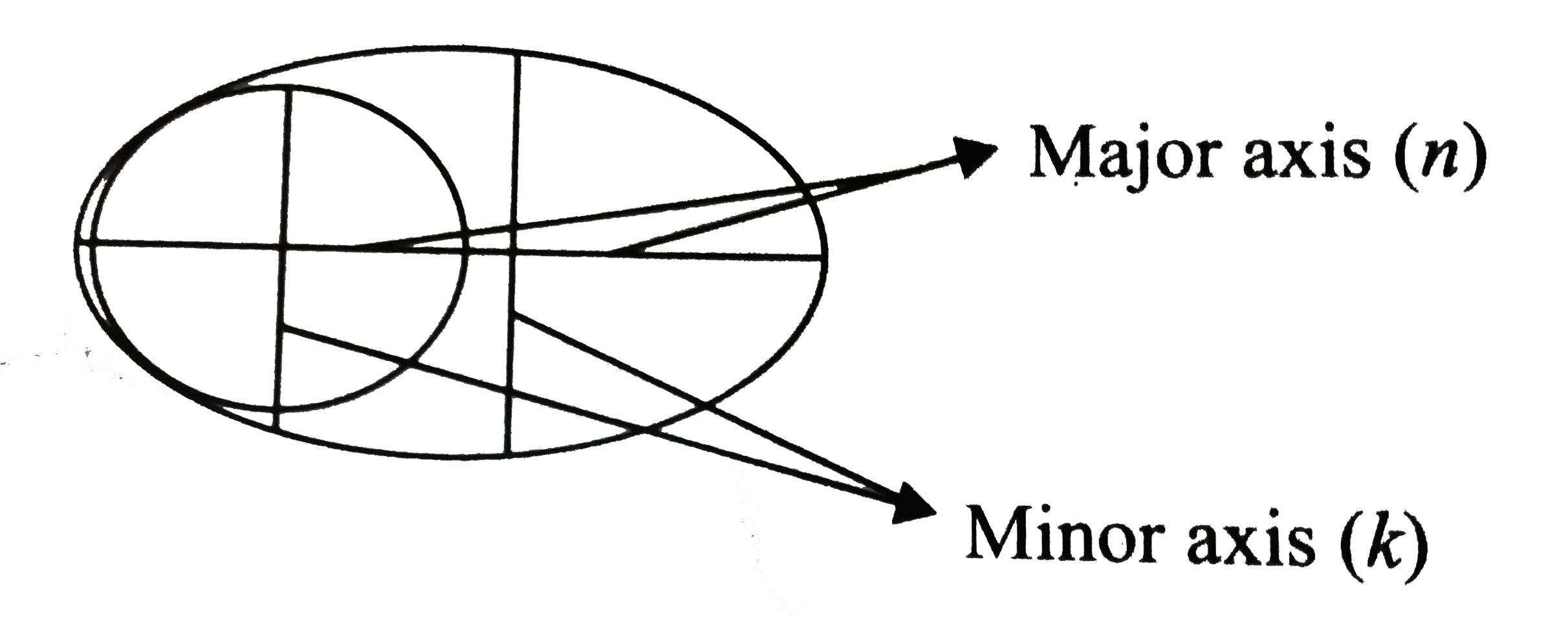
If n is the major axis and k is th e minor axis , then n/k=1 for circular shape white n/k>1 for elliptical shape
Which orbit shape has highest n/k>>1 value?
7s
5p
3d
4d
The correct Answer is:C
3d
Topper's Solved these Questions
- ATOMIC STRUCTUREBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - Exercises Multiple Correct
45 Videos
- ATOMIC STRUCTUREBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - Exercises Single Correct
127 Videos
- ATOMIC STRUCTUREBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - Exercises (Subjective)
52 Videos
- APPENDIX - INORGANIC VOLUME 1BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - APPENDIX - INORGANIC VOLUME 1EXERCISE - chapter-7 Single correct answer
1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - Archives Subjective
15 Videos
Similar Questions
The shape of orbitals are related to the ratio of principal quantum number (n) to substiary quantum number (k,a modifacation of Bohr-sommerfield theory ).The value of k for any shell has a value ranging betwe3en n to l .The amximum value for k is given for x sub-shell white k becomes with p, d,f........ repectively upto minimum value
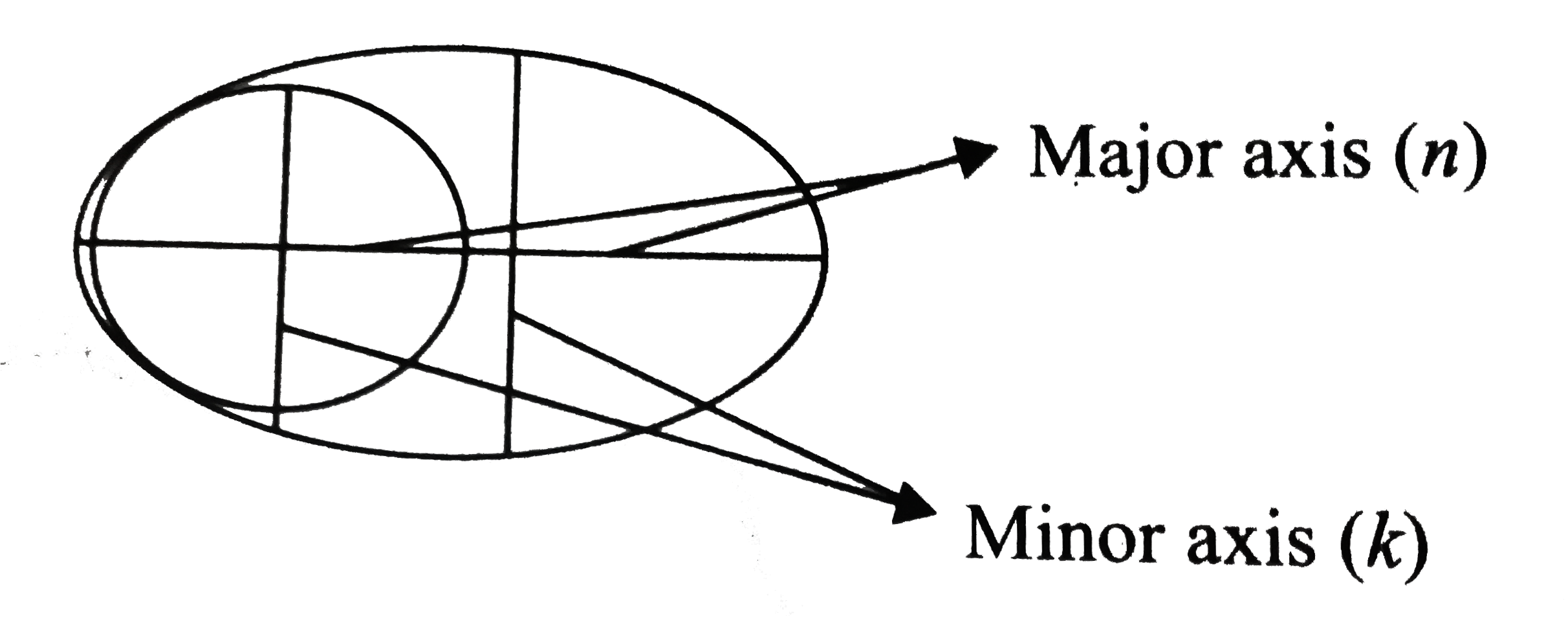
If n is the major axis and k is th e minor axis , then n/k=1 for circular shape white n/k>1 for elliptical shape
Which shape is used to be circular having n/k value
View SolutionThe shape of orbitals are related to the ratio of principal quantum number (n) to substiary quantum number (k,a modifacation of Bohr-sommerfield theory ).The value of k for any shell has a value ranging betwe3en n to l .The amximum value for k is given for x sub-shell white k becomes with p, d,f........ repectively upto minimum value
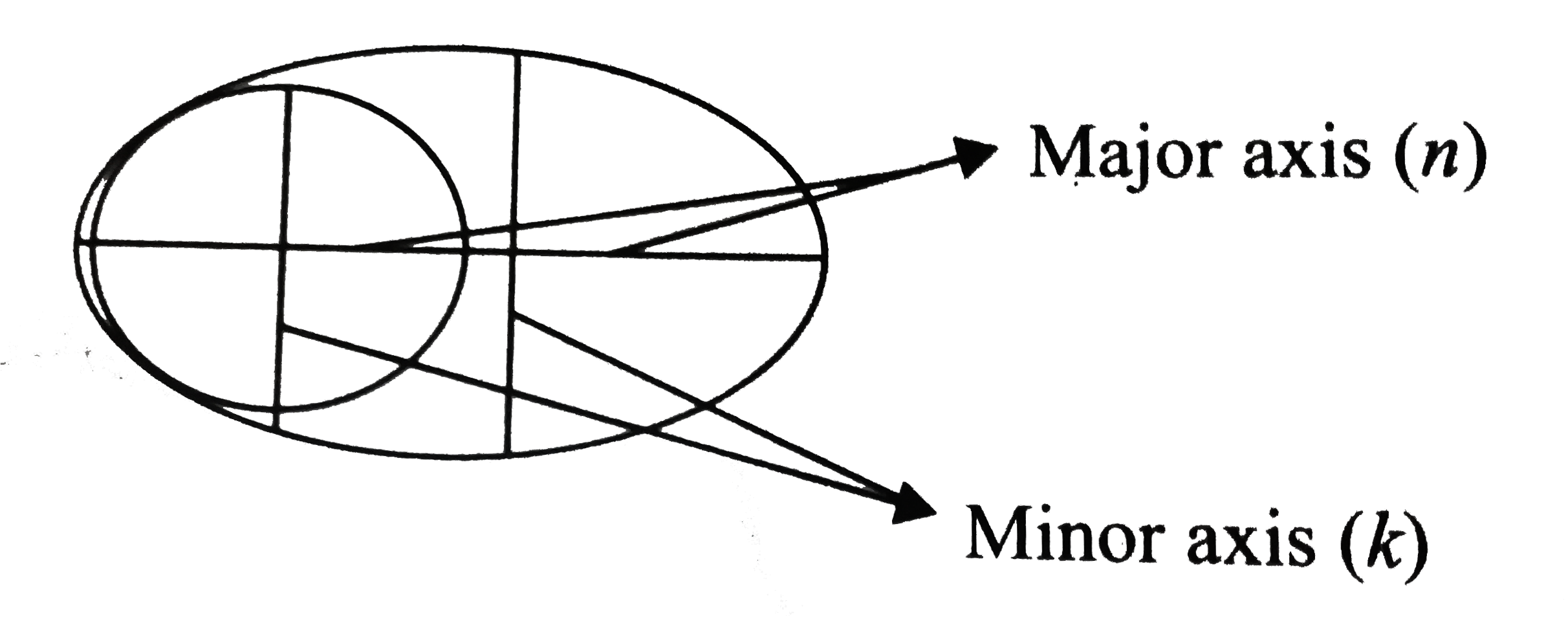
If n is the major axis and k is th e minor axis , then n/k=1 for circular shape white n/k>1 for elliptical shape
Which value of n and k suggest about the shape of 3s orbitsl?
View SolutionThe shape of orbitals are related to the ratio of principal quantum number (n) to substiary quantum number (k,a modifacation of Bohr-sommerfield theory ).The value of k for any shell has a value ranging betwe3en n to l .The amximum value for k is given for x sub-shell white k becomes with p, d,f........ repectively upto minimum value
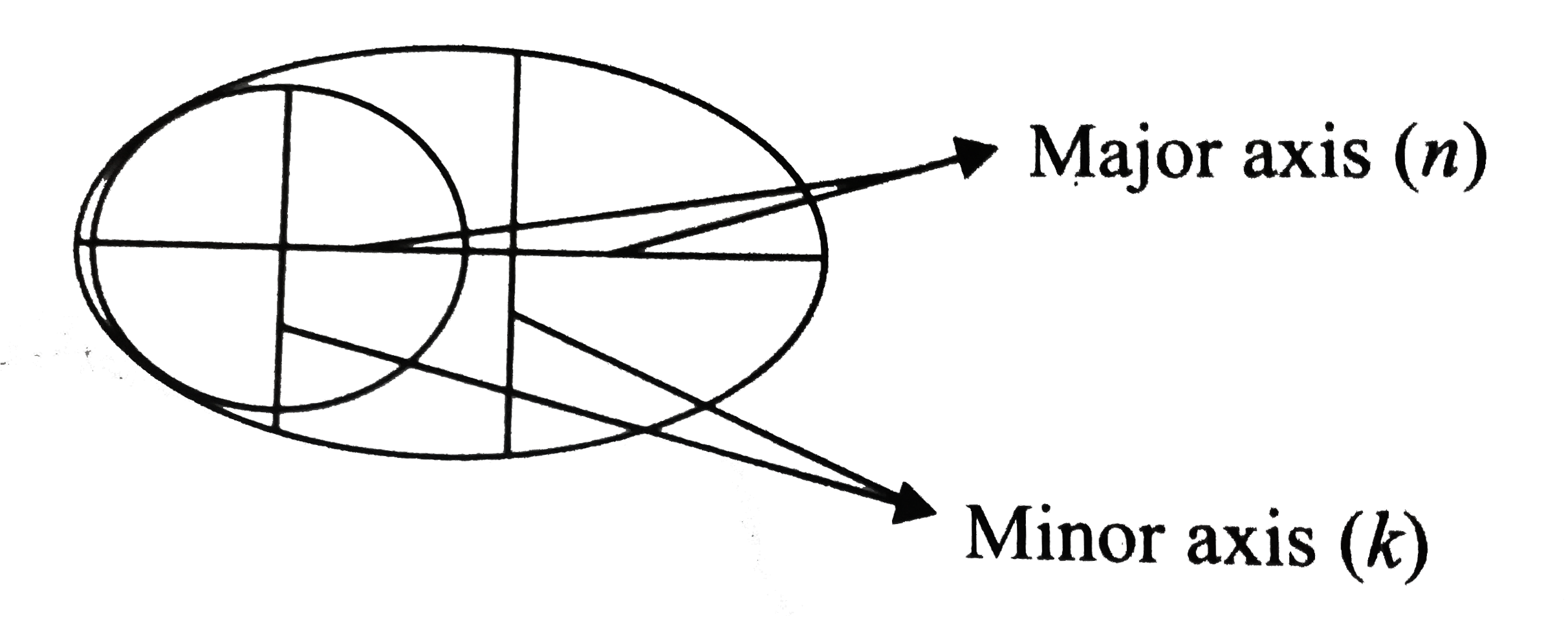
If n is the major axis and k is th e minor axis , then n/k=1 for circular shape white n/k>1 for elliptical shape
Which is correct according to the increasing elliptical number of sub-shell ?
View SolutionThe principal quantum number, n describes
View SolutionPrincipal quantum number is related to
View SolutionWhat are the values of principal quantum number (n) and azimuthal quantum number (l) for a 3d electron ?
View SolutionThe total number of orbitals in a shell having principal quantum n is
View SolutionThe principal quantum number of an atom is related in the
View SolutionThe principal quantum number of an atom is related in the
View SolutionThe principal quantum number of an atom is related in the
View Solution

