Chemical reactions are invariably associated with the transfter of energy either in the form of heat or light. In the laboratory, heat changes in physical and chemical processes are measured with an instrument called calorimeter. Heat change in the process is calculated as
q=msΔTs=Specific heat=cΔTc=Heat capacity
Heat of reaction at constant volume is measured using bomb calorimeter.
qV=ΔU= Internal energy change
Heat of reaction at constant pressure is measured using simple or water calorimeter.
qp=ΔH
qp=qV+PΔV
ΔH=ΔU+ΔnRT
The heat capacity of a bomb calorimeter is 500JK−1. When 0.1g of methane was burnt in this calorimeter, the temperature rose by 2∘C. The value of ΔU per mole will be
+2kJ
−2kJ
+260kJ
−160kJ
To solve the problem, we need to calculate the change in internal energy (ΔU) per mole of methane (CH₄) burned in a bomb calorimeter. Here's the step-by-step solution:
Step 1: Calculate the heat released using the calorimeter's heat capacity
The heat released (q) can be calculated using the formula:
q=CΔT
where:
- C is the heat capacity of the calorimeter (500 J/K)
- ΔT is the change in temperature (2.2 °C)
Substituting the values:
q=500J/K×2.2K=1100J
Step 2: Determine the amount of heat released per gram of methane
We know that 0.1 g of methane was burned, which released 1100 J. To find the heat released per gram of methane, we can use the following relationship:
Heat released per gram=qmass of CH4
Substituting the values:
Heat released per gram=1100J0.1g=11000J/g
Step 3: Calculate the heat released per mole of methane
The molar mass of methane (CH₄) is approximately 16 g/mol. To find the heat released per mole, we multiply the heat released per gram by the molar mass:
Heat released per mole=11000J/g×16g/mol=176000J/mol
Step 4: Convert the heat released to kilojoules
Since we typically express energy in kilojoules, we convert the value:
176000J/mol=176kJ/mol
Step 5: Determine the sign of ΔU
Since the reaction is exothermic (heat is released), we express ΔU as a negative value:
ΔU=−176kJ/mol
Final Answer
The value of ΔU per mole for the combustion of methane is:
ΔU=−176kJ/mol
---
The heat released (q) can be calculated using the formula:
q=CΔT
where:
- C is the heat capacity of the calorimeter (500 J/K)
- ΔT is the change in temperature (2.2 °C)
q=500J/K×2.2K=1100J
We know that 0.1 g of methane was burned, which released 1100 J. To find the heat released per gram of methane, we can use the following relationship:
Heat released per gram=qmass of CH4
Heat released per gram=1100J0.1g=11000J/g
The molar mass of methane (CH₄) is approximately 16 g/mol. To find the heat released per mole, we multiply the heat released per gram by the molar mass:
Heat released per mole=11000J/g×16g/mol=176000J/mol
Since we typically express energy in kilojoules, we convert the value:
176000J/mol=176kJ/mol
Since the reaction is exothermic (heat is released), we express ΔU as a negative value:
ΔU=−176kJ/mol
The value of ΔU per mole for the combustion of methane is:
ΔU=−176kJ/mol
Topper's Solved these Questions
- THERMODYNAMICSBook:CENGAGE CHEMISTRY ENGLISHChapter:THERMODYNAMICSExercise:Paragraph for Problem
Explore 1 Video
- THERMODYNAMICSBook:CENGAGE CHEMISTRY ENGLISHChapter:THERMODYNAMICSExercise:Exercises (Multiple Correct)
Explore 50 Videos
- THERMODYNAMICSBook:CENGAGE CHEMISTRY ENGLISHChapter:THERMODYNAMICSExercise:Exercises (Subjective)
Explore 68 Videos
- STOICHIOMETRYBook:CENGAGE CHEMISTRY ENGLISHChapter:STOICHIOMETRYExercise:Archives Subjective
Explore 33 Videos
Similar Questions
Three moles of CO2 gas expands isothermally (in thermal contact with the surroundings, temperature =15.0∘C) against a fixed external pressure of 1.00 bar. The initial and final volumes of the gas are 10.0L and 30.0L, respectively. ltbr> Change in entropy of the universe is
View SolutionSelect the correct statement
View SolutionEthylene on combustion gives carbon dioxide and water. Its heat of combustion is 1410.0kJmol−1. If the heat of formation of CO2 and H2O are 393.3kJ and 286.2kJ, respectively. Calculate the heat of formation of ethylene.
View SolutionIn the analysis of 0.1 of sample of feldspar, 0.118 g of mixture of NaCl and KCl is obtained which on treatment with AgNO3 gives 0.2451 of AgCl. Calculate the precentage of Na2O and K2O in feldspar.
View SolutionChemical reactions are invariably associated with the transfter of energy either in the form of heat or light. In the laboratory, heat changes in physical and chemical processes are measured with an instrument called calorimeter. Heat change in the process is calculated as {:(q = ms DeltaT,,s ="Specific heat"),(=cDeltaT,,c ="Heat capacity"):} Heat of reaction at constant volume is measured using bomb calorimeter. q_(V) = DeltaU = Internal energy change Heat of reaction at constant pressure is measured using simple or water calorimeter. q_(p) = DeltaH q_(p) = q_(V) +P DeltaV DeltaH = DeltaU +DeltanRT The heat capacity of a bomb calorimeter is 500 JK^(-1) . When 0.1g of methane was burnt in this calorimeter, the temperature rose by 2^(@)C . The value of DeltaU per mole will be
View SolutionChemical reactions are invariably associated with the transfter of energy either in the form of heat or light. In the laboratory, heat changes in physical and chemical processes are measured with an instrument called calorimeter. Heat change in the process is calculated as
{:(q = ms DeltaT,,s =Specific heat),(=cDeltaT,,c =Heat capacity):}<br>Heatofreactionatconstantvolumeismeasuredusingbombcalorimeter.<br>q_(V) = DeltaU =Internale≠rgychan≥<br>Heatofreactionatconstantpressureismeasuredusingsimp≤orwatercalorimeter.<br>q_(p) = DeltaH<br>q_(p) = q_(V) +P DeltaV<br>DeltaH = DeltaU +DeltanRT<br>ForwhichreactionwillDeltaH = DeltaU? Assume each reaction is carried out in an open container.
View SolutionChemical reactions are invariably associated with the transfter of energy either in the form of heat or light. In the laboratory, heat changes in physical and chemical processes are measured with an instrument called calorimeter. Heat change in the process is calculated as {:(q = ms DeltaT,,s ="Specific heat"),(=cDeltaT,,c ="Heat capacity"):} Heat of reaction at constant volume is measured using bomb calorimeter. q_(V) = DeltaU = Internal energy change Heat of reaction at constant pressure is measured using simple or water calorimeter. q_(p) = DeltaH q_(p) = q_(V) +P DeltaV DeltaH = DeltaU +DeltanRT The enthalpy of fusion of ice is 6.02 kJ mol^(-1) . The heat capacity of water is 4.18 J g^(-1)C^(-1) . What is the smallest number of ice cubes at 0^(@)C , each containing one molw of water, the are needed to cool 500g of liquid water from 20^(@)C to 0^(@)C ?
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as: q-msDeltaT," "s= specific heat =cDeltaT," "c= Heat capacity. Heat of reaction at constant pressure is measured using bomb calorimeter. q_(V)=DeltaU= Internal energy change heat of reaction at constant pressure is measrued using simple or water calorimeter. q_(P)=DeltaH q_(P)=q_(V)+PDeltaV DeltaH=DeltaU+DeltanRT The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation: (DeltaH_(2)-DeltaH_(1))/(T_(2)-T_(1))=DeltaC_(P)," "(DeltaU_(2)-DeltaU_(1))/(T_(2)-T_(1))=DeltaC_(V) . Q. Matht eh List-I and List-II and select the answer from the given codes:
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as:
q−msΔT, s=specific heat
=cΔT, c= Heat capacity.
Heat of reaction at constant pressure is measured using bomb calorimeter.
qV=ΔU= Internal energy change
heat of reaction at constant pressure is measrued using simple or water calorimeter.
qP=ΔH
qP=qV+PΔV
ΔH=ΔU+ΔnRT
The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation:
ΔH2−ΔH1T2−T1=ΔCP, ΔU2−ΔU1T2−T1=ΔCV.
Q. The heat capacity of a bomb calorimeter is 500 J/K. When 0.1g of methane was burnt in this calorimeter, the temperature rose by 2∘C. the value of ΔU per mole will be:
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as:
q−msΔT, s=specific heat
=cΔT, c= Heat capacity.
Heat of reaction at constant pressure is measured using bomb calorimeter.
qV=ΔU= Internal energy change
heat of reaction at constant pressure is measrued using simple or water calorimeter.
qP=ΔH
qP=qV+PΔV
ΔH=ΔU+ΔnRT
The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation:
ΔH2−ΔH1T2−T1=ΔCP, ΔU2−ΔU1T2−T1=ΔCV.
Q.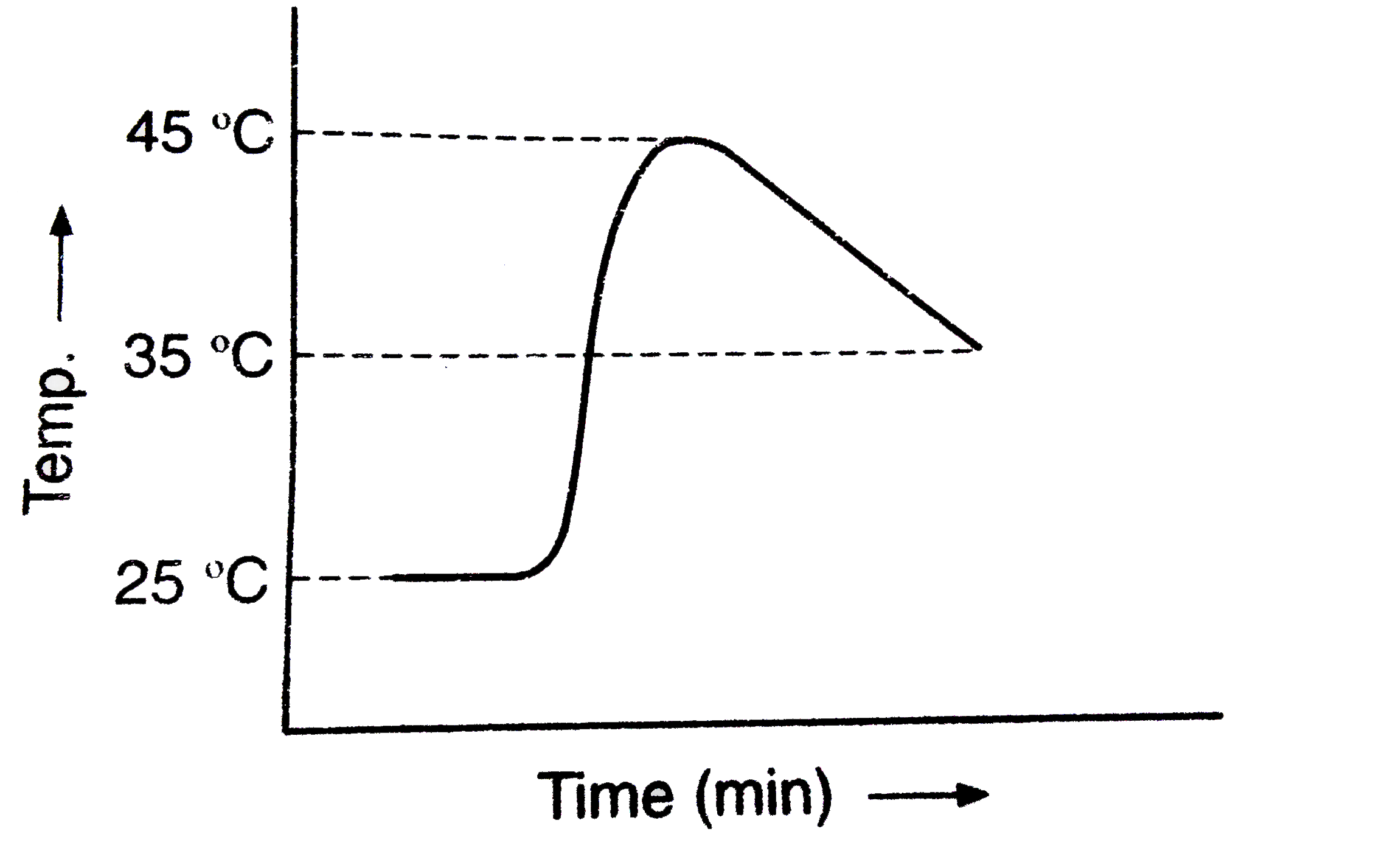
What value of ΔT should be used for the calorimetry experiment that gives the following graphical result?
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as:
q−msΔT, s=specific heat
=cΔT, c= Heat capacity.
Heat of reaction at constant pressure is measured using bomb calorimeter.
qV=ΔU= Internal energy change
heat of reaction at constant pressure is measrued using simple or water calorimeter.
qP=ΔH
qP=qV+PΔV
ΔH=ΔU+ΔnRT
The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation:
ΔH2−ΔH1T2−T1=ΔCP, ΔU2−ΔU1T2−T1=ΔCV.
Q. The enthalpy of fusion of ice is 6.02 kJ mol−1. The heat capacity of water is 4.18Jg−1.∘C−1. What is the smallest number of ice cubes at 0∘C, each containing one mole of water, that are needed to cool 500 g of liquid water from 20C to 0∘C?
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as:
q−msΔT, s=specific heat
=cΔT, c= Heat capacity.
Heat of reaction at constant pressure is measured using bomb calorimeter.
qV=ΔU= Internal energy change
heat of reaction at constant pressure is measrued using simple or water calorimeter.
qP=ΔH
qP=qV+PΔV
ΔH=ΔU+ΔnRT
The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation:
ΔH2−ΔH1T2−T1=ΔCP, ΔU2−ΔU1T2−T1=ΔCV.
Q. The enthalpy change (ΔH) for the reaction,
N2(g)+3H2(g)→2NH2(g)
is -91.38 kJ at 298 K. The nternal energy change ΔU at 298K is:
View SolutionChemical reactions are invariable associated with the transfer of enery either in the form of heat or light. In the laboratory, heat energy I physical and chemical processes are measured with an instrument called caloriemter. Heat change in the process is calcualted as: q-msDeltaT," "s= specific heat =cDeltaT," "c= Heat capacity. Heat of reaction at constant pressure is measured using bomb calorimeter. q_(V)=DeltaU= Internal energy change heat of reaction at constant pressure is measrued using simple or water calorimeter. q_(P)=DeltaH q_(P)=q_(V)+PDeltaV DeltaH=DeltaU+DeltanRT The amount of energy released during a chemical change depends on the physical state of reactants and products, te conditions of pressure, temperatrue and volume at which the reaction is carried out. the variation of heat of reaction with temperature and pressure is given by kirchhoff's equation: (DeltaH_(2)-DeltaH_(1))/(T_(2)-T_(1))=DeltaC_(P)," "(DeltaU_(2)-DeltaU_(1))/(T_(2)-T_(1))=DeltaC_(V) . Q. The specific heats of I_(2) in vapour and solid states are 0.031 and 0.055 cal/g respectively. The heat of sublimation of iodine at 200^(@)C is 6.096 kcal mol^(-1) . the heat of sublimation of iodine at 250^(@)C will be:
View SolutionThe heat measured for a reaction in a bomb calorimeter is
View SolutionIn a bomb calorimeter, the heat of reaction is measured at :
View SolutionThe heat measured for a reaction in a bomb calorimeter is
View Solution
