The degree of dissociation for PtCl4 complex is 70%. Calculate the Van't Hoff factor.
To calculate the Van't Hoff factor (i) for the PtCl4 complex with a degree of dissociation of 70%, we can follow these steps:
Step 1: Understand the Degree of Dissociation
The degree of dissociation (α) is given as 70%, which can be expressed as:
α=0.7
Step 2: Write the Dissociation Reaction
The dissociation of the PtCl4 complex can be represented as:
PtCl4→Pt4++4Cl−
Step 3: Set Up Initial and Equilibrium Moles
Assume we start with 1 mole of PtCl4. The initial moles are:
- PtCl4: 1 mole
- Pt4+: 0 moles
- Cl-: 0 moles
At equilibrium, if α moles of PtCl4 dissociate, we have:
- PtCl4: 1−α moles
- Pt4+: α moles
- Cl-: 4α moles
Step 4: Calculate the Total Moles at Equilibrium
The total moles at equilibrium can be calculated as:
Total moles=(1−α)+α+4α
Simplifying this gives:
Total moles=1+4α
Step 5: Substitute the Value of α
Substituting α = 0.7 into the equation:
Total moles=1+4(0.7)=1+2.8=3.8
Step 6: Calculate the Van't Hoff Factor (i)
The Van't Hoff factor (i) is defined as the total moles at equilibrium divided by the initial moles:
i=Total moles at equilibriumInitial moles=3.81=3.8
Final Answer
Thus, the Van't Hoff factor (i) for the PtCl4 complex is:
3.8
---
The degree of dissociation (α) is given as 70%, which can be expressed as:
α=0.7
The dissociation of the PtCl4 complex can be represented as:
PtCl4→Pt4++4Cl−
Assume we start with 1 mole of PtCl4. The initial moles are:
- PtCl4: 1 mole
- Pt4+: 0 moles
- Cl-: 0 moles
- PtCl4: 1−α moles
- Pt4+: α moles
- Cl-: 4α moles
The total moles at equilibrium can be calculated as:
Total moles=(1−α)+α+4α
Simplifying this gives:
Total moles=1+4α
Substituting α = 0.7 into the equation:
Total moles=1+4(0.7)=1+2.8=3.8
The Van't Hoff factor (i) is defined as the total moles at equilibrium divided by the initial moles:
i=Total moles at equilibriumInitial moles=3.81=3.8
Thus, the Van't Hoff factor (i) for the PtCl4 complex is:
3.8
Topper's Solved these Questions
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercises (Linked Comprehension)
Explore 57 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercises (Multiple Correct)
Explore 25 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Ex 2.3 (Objective)
Explore 9 Videos
- SOLID STATEBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLID STATEExercise:Ex 1.2 (Objective)
Explore 9 Videos
- SURFACE CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:SURFACE CHEMISTRYExercise:Archives Subjective
Explore 2 Videos
Similar Questions
An aqueous solution freezes at 272.4 K while pure water freezes at 273 K. Given Kf=1.86Kkgmol−1,Kb=0.512Kkgmol−1 and vapour pressure of water at 298 K = 23.756 mm Hg. Determine the following.
Molality of the solution is
View SolutionTwo miscible liquids A and B having vapour pressure in pure state P∘A and P∘B are mixed in mole fraction χA and χB to get a mixtue having total vapour vapour pressure of mixture PM. Which of the following relations are correct?
View SolutionThe molal elevation constant of water =0.52Km−1. The boiling point of 1.0molal aqueous KCl solution (assuming complete dissociation of KCl) should be
View SolutionThe structure shown here represents
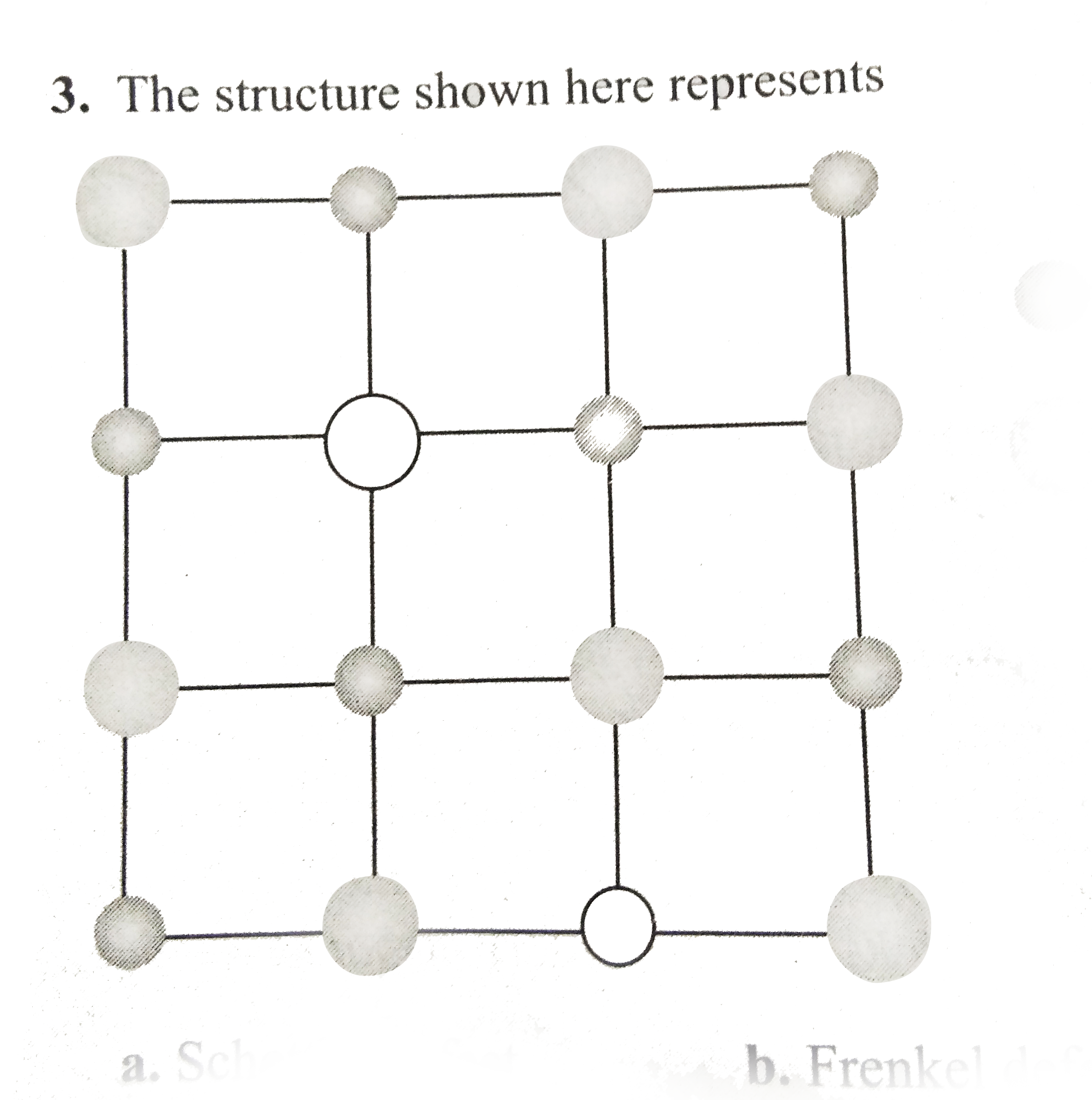
View SolutionTwenty precent of the surface sites of a catalyst is occupied by nitrogen molecules. The density of surface sites is 6.023×1014cm−2 . The total sarface area is 1000cm2 . The catalyst is is henced to 300K and nitrogen is completely desorbed a pressure of 0.001 atm and volume of 2.46cm3 . Calculate the number of sites occupied by niitrogen molecules.
View SolutionThe degree of dissociation for K4[Fe(CN)6] is 60%. Calculate the Van't Hoff factor.
View SolutionThe degree of association is 70% for the following reaction . Calculate the Van't Hoff factor.
View SolutionThe Van't Hoff factor of a 0.1MAl2(SO4)3 solution is 4.20. The degree of dissociation is
View SolutionThe van't Hoff factor of NaCl assuming 100% dissociation is:
View SolutionIf α is the degree of dissociation of Na2SO4 , the Van't Hoff factor (i) used for calculating molecular mass is :
View SolutionDefine van't Hoff factor . How is it related to the degree of dissociation ?
View SolutionRelationship between van't Hoff ' s factor (i) and degree of dissociation (α) is
View SolutionHow is degree of dissociation and degree of association are related with van't Hoff factor ?
View SolutionIf alpha is the degree of dissociation of Na_(2) SO_(4) , the van't Hoff 's factor (i) used for calculating the molecular mass is _______.
View SolutionDerive the relationship between degree of dissociation and dissociation constant in weak electrolytes. (any one)
View SolutionThe degree of dissociation for K4[Fe(CN)6] is 60%. Calculate the Van't Hoff factor.
View Solution
