A maxima or minima is obtained in the temperature composition curve of a mixture of two liquids indicates
That the liquids are immiscible with one another
That the liquids are partially miscible at the maximum or minimum.
An azeotropic mixture.
A eutectic formation.
The correct Answer is:A, B, D
To solve the question regarding what a maxima or minima in the temperature-composition curve of a mixture of two liquids indicates, we can follow these steps:
1. Understanding Temperature-Composition Curves:
- The temperature-composition curve is a graphical representation that shows how the boiling point of a mixture changes with varying composition of the components in the mixture.
Hint: Recall that these curves are used to analyze the behavior of liquid mixtures when they are heated.
2. Identifying Maxima and Minima:
- In a temperature-composition curve, a maxima indicates a point where the boiling point is at its highest for a given composition, while a minima indicates a point where the boiling point is at its lowest.
Hint: Think about what happens to the boiling point when different components are mixed.
3. Analyzing the Implications of Maxima and Minima:
- The presence of maxima and minima in the curve suggests that the components of the mixture interact in a specific way, leading to distinct boiling points based on their ratio.
Hint: Consider how interactions between molecules can affect their physical properties, such as boiling points.
4. Determining the Type of Mixture:
- A mixture that shows maxima and minima in its temperature-composition curve is indicative of an azeotropic mixture. Azeotropic mixtures boil at a constant temperature and maintain a fixed composition in the vapor phase.
Hint: Recall the definition of azeotropic mixtures and how they differ from ideal solutions.
5. Conclusion:
- Therefore, the presence of maxima and minima in the temperature-composition curve of a mixture of two liquids indicates that the mixture is an azeotropic mixture.
Hint: Summarize the characteristics of azeotropic mixtures to reinforce your understanding.
Final Answer:
A maxima or minima in the temperature-composition curve of a mixture of two liquids indicates that the mixture is an azeotropic mixture.
- The temperature-composition curve is a graphical representation that shows how the boiling point of a mixture changes with varying composition of the components in the mixture.
- In a temperature-composition curve, a maxima indicates a point where the boiling point is at its highest for a given composition, while a minima indicates a point where the boiling point is at its lowest.
- The presence of maxima and minima in the curve suggests that the components of the mixture interact in a specific way, leading to distinct boiling points based on their ratio.
- A mixture that shows maxima and minima in its temperature-composition curve is indicative of an azeotropic mixture. Azeotropic mixtures boil at a constant temperature and maintain a fixed composition in the vapor phase.
- Therefore, the presence of maxima and minima in the temperature-composition curve of a mixture of two liquids indicates that the mixture is an azeotropic mixture.
A maxima or minima in the temperature-composition curve of a mixture of two liquids indicates that the mixture is an azeotropic mixture.
Topper's Solved these Questions
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercises (Single Correct)
Explore 99 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercise (Assertion-Reasoning)
Explore 18 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercises (Linked Comprehension)
Explore 57 Videos
- SOLID STATEBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLID STATEExercise:Ex 1.2 (Objective)
Explore 9 Videos
- SURFACE CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:SURFACE CHEMISTRYExercise:Archives Subjective
Explore 2 Videos
Similar Questions
The use of common salts, e.g., NaCl or CaCl2 anhydrous, is made to clear snow on the roads. This causes:
View SolutionAssertion (A): The dissolution of gases in water is always an endothermic process.
Reason (R ) : The dissolution of gases in water proceed with a negative value of ΔS.
View SolutionAn aqueous solution freezes at 272.4 K while pure water freezes at 273 K. Given Kf=1.86Kkgmol−1,Kb=0.512Kkgmol−1 and vapour pressure of water at 298 K = 23.756 mm Hg. Determine the following.
Molality of the solution is
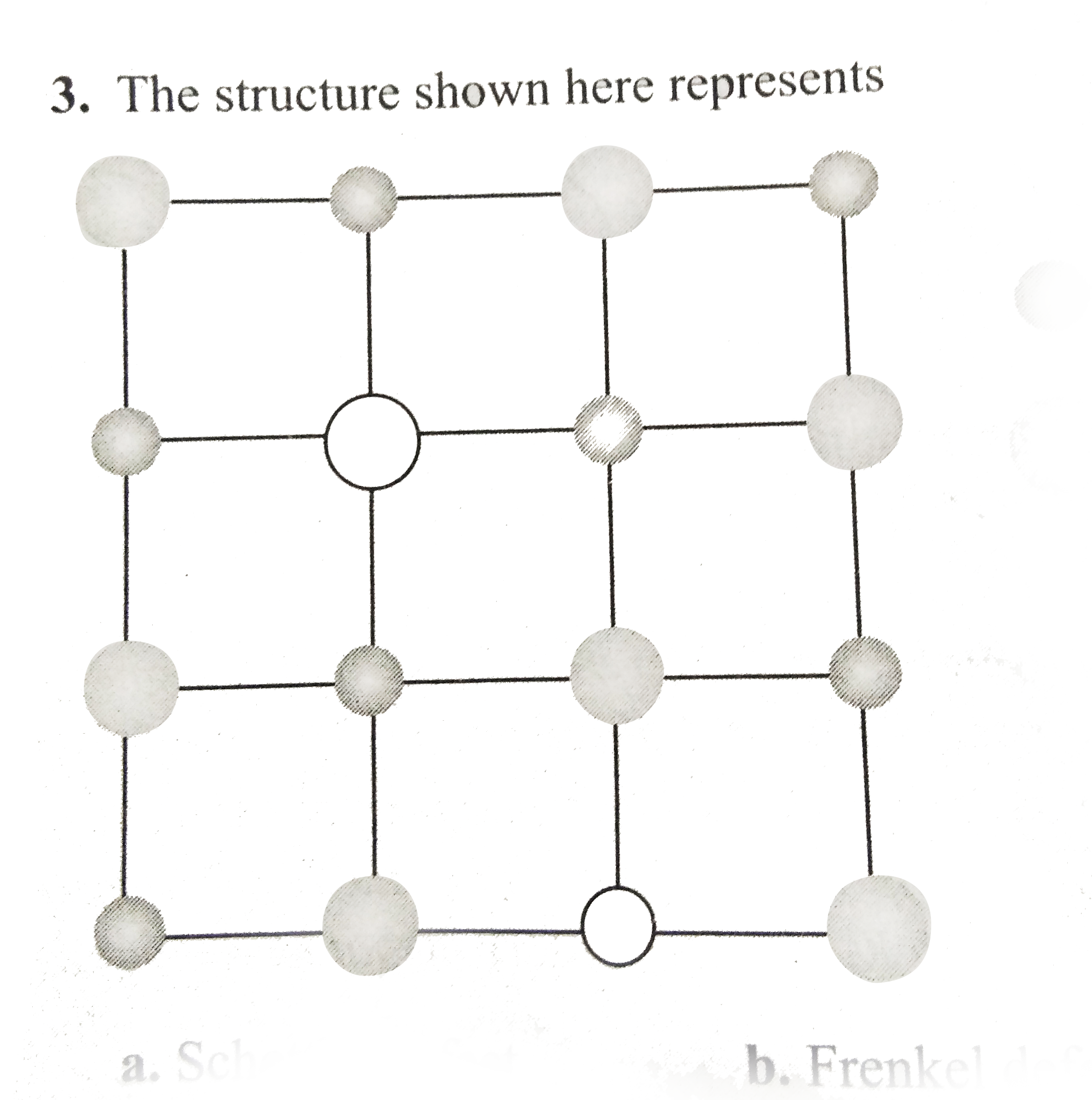
View SolutionThe structure shown here represents
View SolutionTwenty precent of the surface sites of a catalyst is occupied by nitrogen molecules. The density of surface sites is 6.023×1014cm−2 . The total sarface area is 1000cm2 . The catalyst is is henced to 300K and nitrogen is completely desorbed a pressure of 0.001 atm and volume of 2.46cm3 . Calculate the number of sites occupied by niitrogen molecules.
View SolutionA maxima or minima is obtained in the temperature. The composition curve of a mixture of two liquids does not indicate
View SolutionA maximum or minima obtained in the temperature, composition curve of a mixture of two liquids indicates:
View SolutionA maxima or minima obtained in the temperature composition curve of a mixture of two liquids indicates:
View SolutionA maxima or minima obtained in the temperature. Composition curve of a mixture of two liquids indicates:
View Solutionशुद्ध द्रव के समान स्थिर ताप पर उबलने तथा संघटन में बिना ________ के आसवित होने वाला दो पूर्णतया मिश्रित द्रवों का विलयन.................... मिश्रण कहलाता है ।
View Solutionदो पूर्णतः मिश्रणीय अनादर्श द्रवों का मिश्रण, जो नियत ताप पर संघटन में परिवर्तन के बिना आसवित हो जाता है जबकि यह शुद्ध द्रव है, इस मिश्रण को कहते हैं
View SolutionThe function f(x)=2x3−3x2−12x−4 has: a)No maxima and minima b)One maxima and one minima c)Two maxima d)Two minima
View SolutionA maxima or minima obtained in the temperature, composition curve of a mixture of two liquids indicates:
View SolutionA maxima or minima obtained in the temperature, composition curve of a mixture of two liquids indicates:
View SolutionMaxima Minima-cubic curves
View Solution
