Assertion (A): Larger the value of cryoscopic constant of the solvent, lesser will be the freezing point of the solution.
Reason (R ): Depression in the freezing point depends on the nature of the solvent.
If both (A) and (R) are correct, and (R) is the correct explanation of (A).
If both (A) and (R) are correct, but (R) is not the correct explanation of (A).
If (A) is correct, but (R) is incorrect.
If (A) is correct, but (R) is correct.
The correct Answer is:A
To solve the given question, we need to analyze both the Assertion (A) and the Reason (R) statements provided.
Step 1: Analyze the Assertion (A)
The assertion states: "Larger the value of cryoscopic constant of the solvent, lesser will be the freezing point of the solution."
- The cryoscopic constant (Kf) is a property of the solvent that indicates how much the freezing point of the solvent will decrease per mole of solute added.
- The relationship is given by the formula:
ΔTf=Kf×m
where ΔTf is the depression in freezing point, Kf is the cryoscopic constant, and m is the molality of the solution.
- If Kf is larger, for a given molality, ΔTf will be larger, meaning the freezing point of the solution will be lower.
- Therefore, the assertion is true.
Step 2: Analyze the Reason (R)
The reason states: "Depression in the freezing point depends on the nature of the solvent."
- Depression in freezing point is a colligative property, which means it depends on the number of solute particles in the solution, not on the nature of the solvent.
- While the cryoscopic constant (Kf) is specific to the solvent, the depression itself is influenced by the amount of solute and not the characteristics of the solvent itself.
- Thus, the reason is false.
Step 3: Conclusion
Based on the analysis:
- The assertion (A) is true.
- The reason (R) is false.
Therefore, the correct conclusion is that the assertion is correct, but the reason is incorrect.
Final Answer: Assertion (A) is true, and Reason (R) is false.
---
The assertion states: "Larger the value of cryoscopic constant of the solvent, lesser will be the freezing point of the solution."
- The cryoscopic constant (Kf) is a property of the solvent that indicates how much the freezing point of the solvent will decrease per mole of solute added.
- The relationship is given by the formula:
ΔTf=Kf×m
where ΔTf is the depression in freezing point, Kf is the cryoscopic constant, and m is the molality of the solution.
- If Kf is larger, for a given molality, ΔTf will be larger, meaning the freezing point of the solution will be lower.
- Therefore, the assertion is true.
The reason states: "Depression in the freezing point depends on the nature of the solvent."
- Depression in freezing point is a colligative property, which means it depends on the number of solute particles in the solution, not on the nature of the solvent.
- While the cryoscopic constant (Kf) is specific to the solvent, the depression itself is influenced by the amount of solute and not the characteristics of the solvent itself.
- Thus, the reason is false.
Based on the analysis:
- The assertion (A) is true.
- The reason (R) is false.
Therefore, the correct conclusion is that the assertion is correct, but the reason is incorrect.
टॉपर्स ने हल किए ये सवाल
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercise (Interger)
Explore 8 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercise(Fill In The Blanks)
Explore 19 Videos
- SOLUTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLUTIONSExercise:Exercises (Single Correct)
Explore 99 Videos
- SOLID STATEBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLID STATEExercise:Ex 1.2 (Objective)
Explore 9 Videos
- SURFACE CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:SURFACE CHEMISTRYExercise:Archives Subjective
Explore 2 Videos
समान प्रश्न
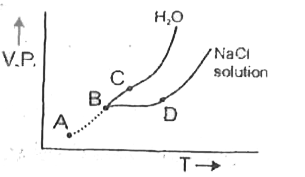
Freezing point of solution is marked as
View SolutionAt freezing point of a solution there is always
View SolutionIn which of the following solution the depression in freezing point is lowest ?
View SolutionThe depression in freezing point is maximum if the solvent used is
View SolutionFor the depression in freezing point experiment, the correct statement(s) is/are:
View SolutionIn the depression of freezing point experiment, it is found that the:
View SolutionWhich of the following will have maximum depression in freezing point ?
View SolutionSolution with highest freezing point is
View SolutionThe freezing point of equimolal solution will be highest for :
View SolutionFreezing point of a solution is smaller than freezing point of a solvent. It is due to :
View Solution
