State True or False
Rusting of Fe is quicker in saline water than in ordinary water.
Presence of Na⊕ and Clc− ions in saline water increases the conductance of the solution in contact with the metal surface, which accelerates the formation of Fe2+0 ion and henc that of rust, Fe2O3.Xh2o.
Topper's Solved these Questions
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRY ENGLISHChapter:NCERT BASED EXERCISEExercise:Conductivity of Ionic Solutions
Explore 8 Videos
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRY ENGLISHChapter:NCERT BASED EXERCISEExercise:Chemical Kinetics (In -Text Questions)
Explore 9 Videos
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRY ENGLISHChapter:NCERT BASED EXERCISEExercise:Short Answer Type Questions(Electrochmical Cell)
Explore 12 Videos
- GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSBook:CENGAGE CHEMISTRY ENGLISHChapter:GRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTSExercise:Exercises Archives (Linked Comprehension)
Explore 1 Video
- NUCLEAR CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:NUCLEAR CHEMISTRYExercise:Archives Subjective
Explore 13 Videos
Similar Questions
Why ac is used in place of dc in measuring the conductance of electrolytes ?
View SolutionFor the reaction R→P, the concentration of a reactant changes from 0.03M to 0.02M in 25 minutes. Calculate the average rate of reaction using units of time both in minutes and seconds.
View SolutionCl2 and Brc− are added to a solution containing 1M each of Clc− and Brc−. What reaction will occur ?
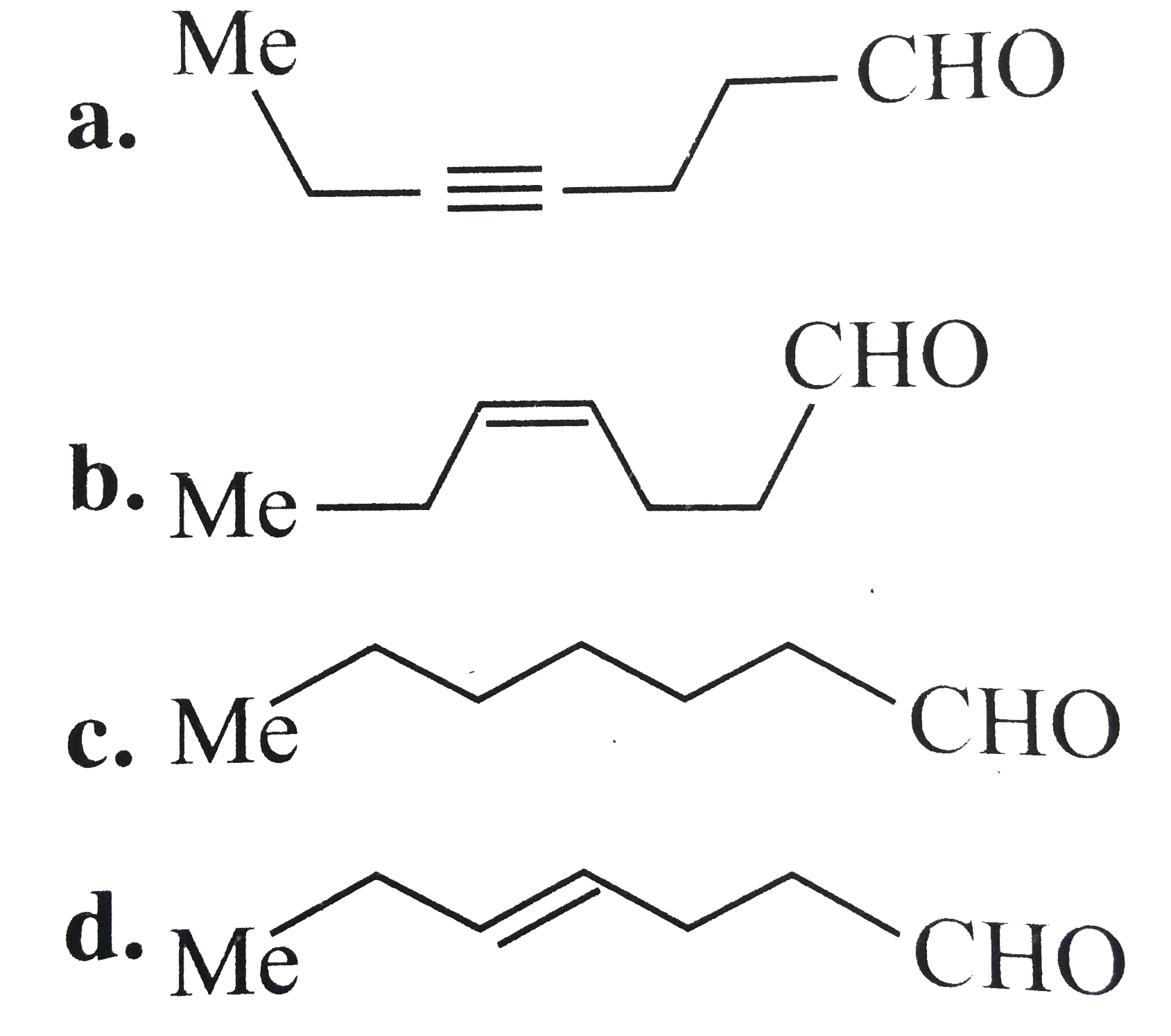
View SolutionIn the following reaction sequence, products I, J, and L are formed. K represents a reagent.
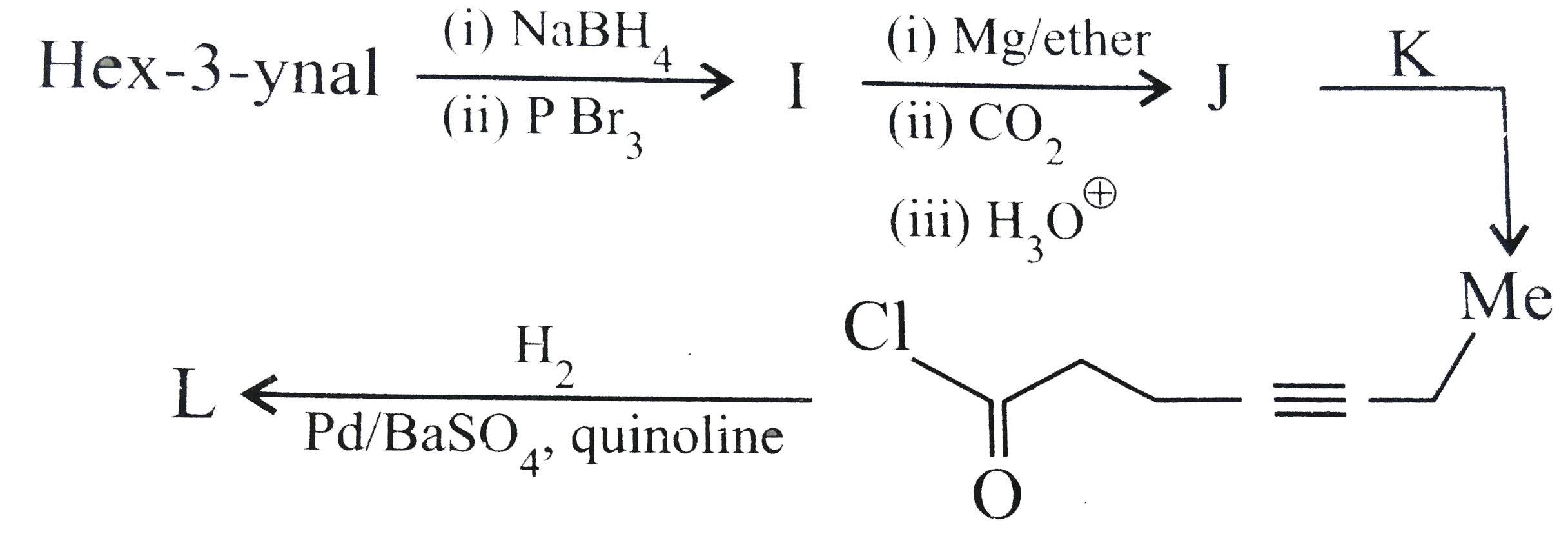
The structures of product I is :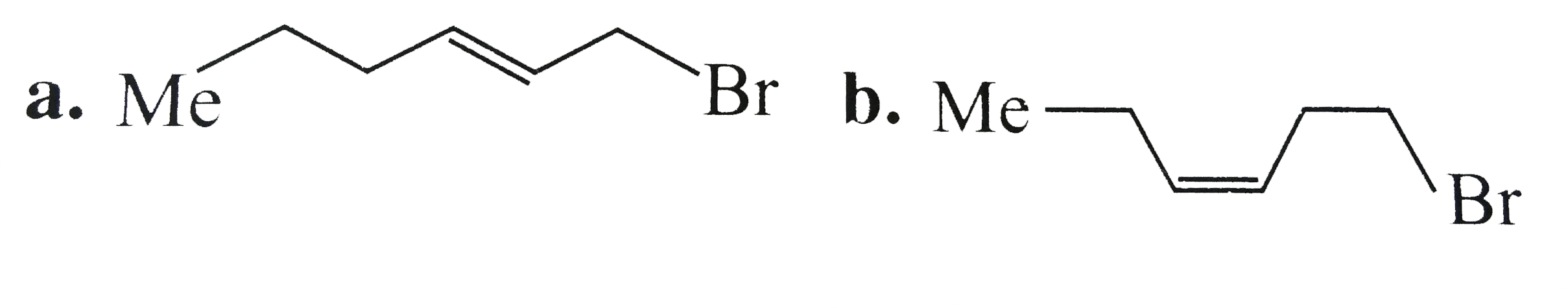
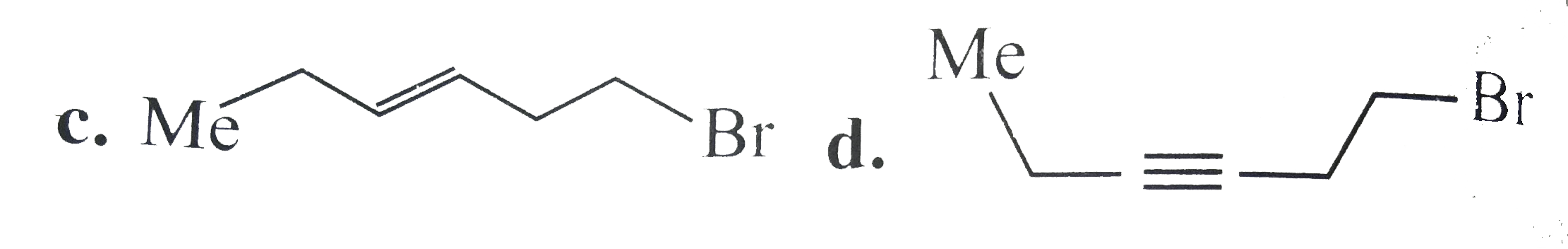
The structures of compounds J and K, respectively, are :
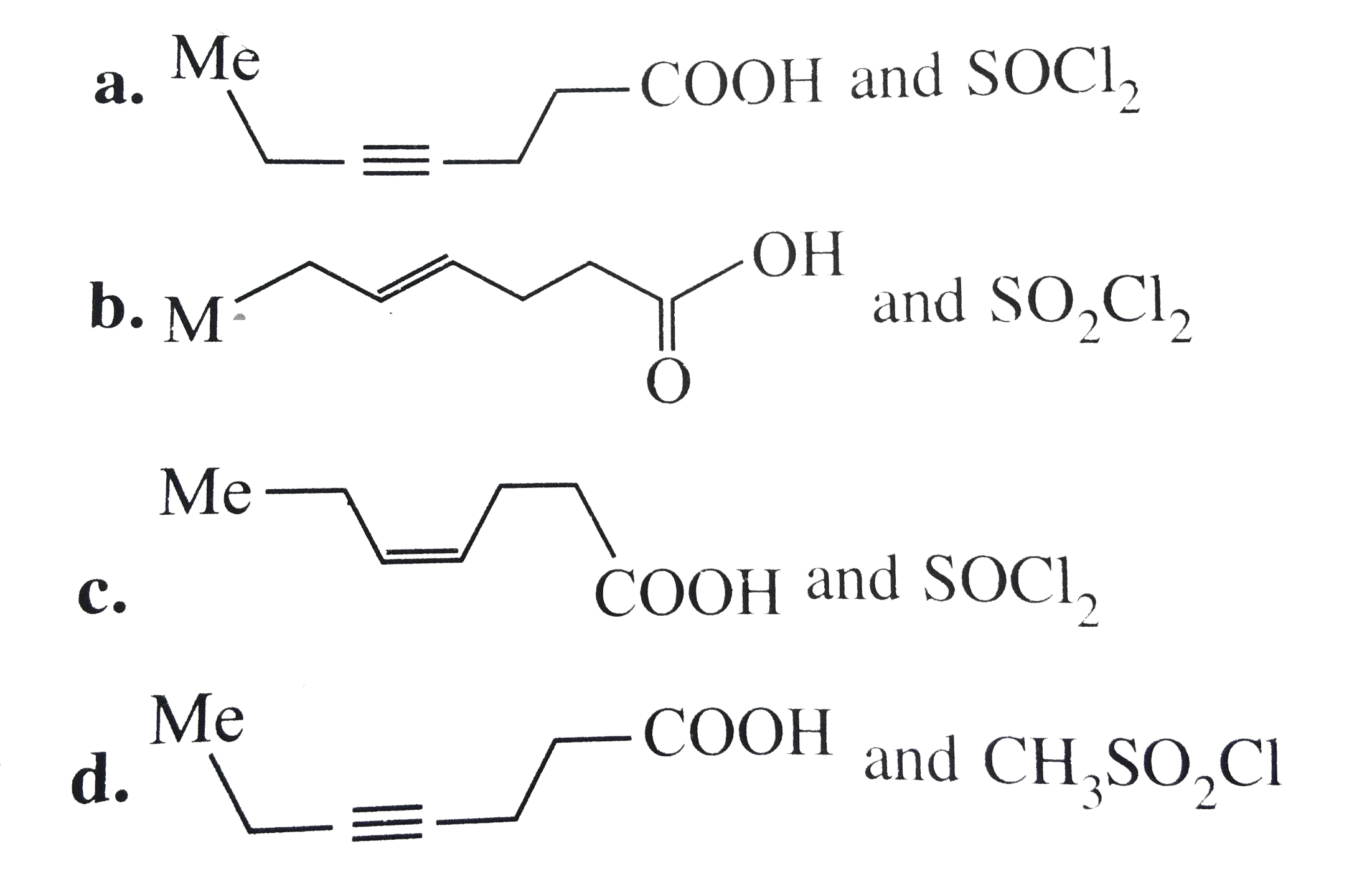
The structure of product L is :
View SolutionRadioactive decay is a first− order process. Radioactive carbon in wood sample decays with a half− life of 5770 years. What is the rate constant ( in years ) for the decay ? What fraction would remains after 11540 years ?
View SolutionRusting of Fe is quicker in saline water than in ordinary water.
View SolutionRusting of Fe is quicker in saline water than in ordinary water. Why ?
View SolutionRusting of iron becomes quicker in saline medium. Explain.
View SolutionRusting of iron is quicker in salline water than in ordinary water. Why is it so?
View SolutionRusting of iron is quicker in saline water than in orderinary water. Give reason.
View Solutionलोहे पर जंग लवणीय जल में तीव्रता से लगती है तथा साधारण जल में नहीं। ऐसा क्यों ?
View Solution(b) (i) Describe about lithium-ion battery and its uses. (ii) Give reasons for the following (1) Rusting of iron is quicker in saline water than in ordinary water. (2) Aluminium metal cannot be produced by the electrolysis of aqueous solution of aluminium salt.
View Solutionসত্য অথবা মিথ্যা লেখো: জল আর বাতাসের অক্সিজেনের সংস্পর্শে লোহায় মরচে পড়ে ৷
View SolutionGive reasons for the following Rusting of iron is quicker in saline water than in ordinary water.
View SolutionGive reasons for the following Rusting of iron is quicker in saline water than in ordinary water. Why is it so?
View Solutionstate whether True or False
Pressure of saline water is greather than purifed water.
View Solution
