Give the IUPAC names for the following polyfunctional compounds:
i. CH3CH2O−CH2−CHOH−CH3
ii. CH3−C∣NO2H−CH2−CH3∣C∣OH−CH3
iii. CH2=H3C∣C−OH∣CH−CH2CN
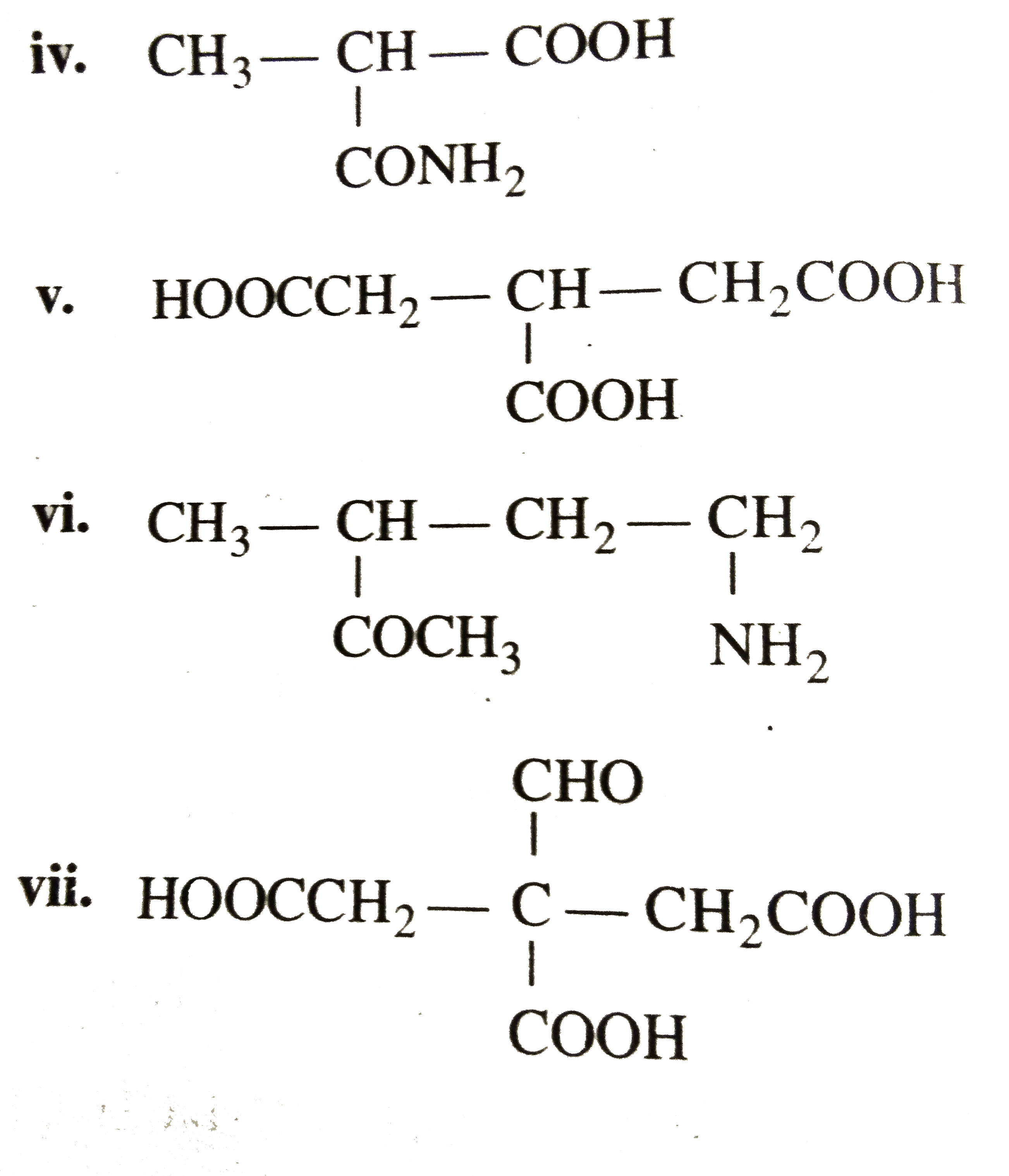
Video Solution
Text Solution
i. 1- Ethoxypropan-2-oal
ii. 2-Methy1-4-nitropentan-2-ol
iii. 3-Hydroxy-4-methypent-4-ene-1nitrile
iv. 2-Carbamoylpropon-1-oic acid
2- carboxamidopropan-1-oic acid
v. Propane-1,2,3-tricarboxlic acid
vi. 5-Amino-3-methypentan-2-one
vii. 2-Formypropane-1,2,3,-tricarboxylic acid
viii. 4-Aminomethy1-2-ethy1-2-methylbutan--al
ix. 2-Hyroxy-4-methy1 hex -5-yn-1-oylchloride
x. Methy1 3-bromo-2-hydroxy-2-methylbutan-1-oate
xi. 3-Hydroxy-4-methylpent-4-en-1-al
|
Topper's Solved these Questions
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSEXERCISE - Solved Example
4 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSEXERCISE - Exercises
10 Videos
- CHEMICAL EQUILIBRIUMBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Subjective type
1 Video
- GENERAL ORGANIC CHEMISTRYBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Analytical and Descriptive
1 Video
Similar Questions
Knowledge Check
The type of isomerism shown by the following compounds is
CH3CH2CH2CH2CH3,CH3−CH3∣CH−CH2CH3,CH3−CH3∣C∣CH3−CH3Aposition isomerismBmetamerismCring-chain isomerismDchain isomerismCH3−CH2−O∣∣C−CH3∣C∣CH3−CH3PhCO3H−−−−−→A,A will be
ACH3−CH2−O−O∣∣C−CH3∣C∣∣CH3−CH3BCH3−CH2−O∣∣C−O−CH3∣C∣CH3−CH3CCH3−CH2−O∣∣C−CH3∣−∣CH3O−CH3DNone of these
Write its IUPAC name
CH3−CH∣CH3−CH2−CH3∣C∣CH2−CH3
View SolutionThe IUPAC name of CH3−C∣CONH2=CH−CH2−O∣∣C∣OH is
View SolutionThe IUPAC name of the compound
CH3CH∣OHCH2−CH3∣4C∣OH−CH3
is
View SolutionThe IUPAC name of the compound
CH3CH∣OHCH2−CH3∣4C∣OH−CH3
is
View SolutionThe monomer of the polymer
~CH2−CH3∣C∣CH3−CH2−CH3∣C∣CH3~
View SolutionGive IUPAC name of:
(i) CH3−CH2−C∣CH2∣CH3H−CH3∣C∣CH3−CH2−OH3
View Solution

