Why is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
For testing of sulphur present in the organic compound lassaigne's extract is acidified with acetic acid (CH3COOH) and not sulphuric acid bacause lead acetate is soluble and does not interfere with the test. If sulphuric acid (H2SO4) is used, lead acetate will react with H2SO4 itself to form white precipitate of PbSO4 as follows
Pb(CH3COO)2+H2HO4→PbSO4⏐↓+2CH3COOH
The white precipitate of PbSO4 so formed will interfere with this test of sulphur.
Pb(CH3COO)2+Na2S→PbS⏐↓+2CH3COONa
Topper's Solved these Questions
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Linked Comprehension Type
Explore 25 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Multiple Correct Answers Type
Explore 10 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:PURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSISExercise:Subjective Type
Explore 11 Videos
- PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYBook:CENGAGE CHEMISTRY ENGLISHChapter:PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRYExercise:Exercises (Archives )Subjective
Explore 4 Videos
- REDOX REACTIONSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDOX REACTIONSExercise:Archives (Integers)
Explore 1 Video
Similar Questions
Qualitative analysis of organic compounds is performed by Lassaigne's test by fusion with metallic sodium, by which the covalent compounds are converted into ionic compounds. Extra elements like N,S,P and halogens are detected by their usual tests.
Q. Which of the following compounds will give positive lassaigne's test for nitrogen
View SolutionWhich of the following statements is/are correct?
View SolutionWhen 100 ml of O2−O3 mixture was passed through turpentine oil, there was reduction of volume by 20 ml. If 100 ml of such a mixture is heated, what will be the increase in the volume?
View SolutionArrange the following in the given order
(a) Decreasing ionic size, Mg2+,O2−,Na⊕,F⊕
(b) Increasing bond length F2,N2,Cl2O2
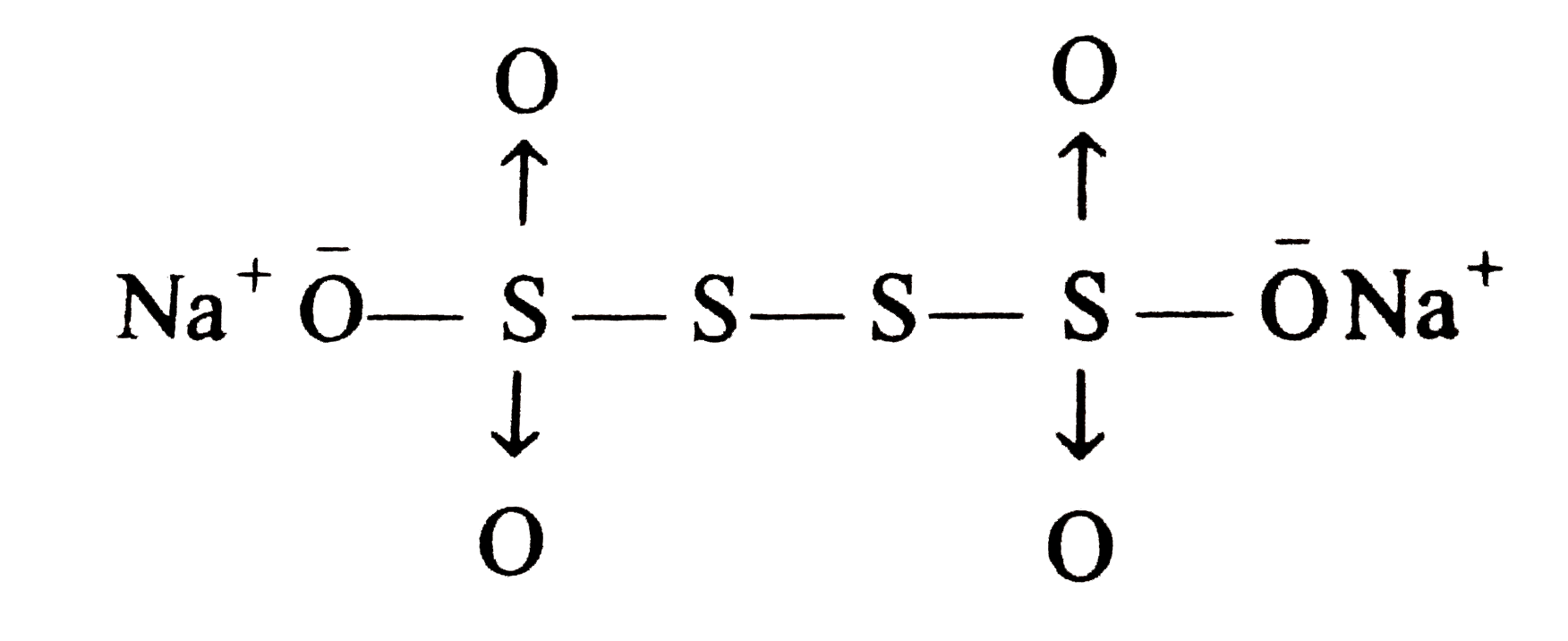
View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:
View SolutionWhy is it necessary to use acetic acid and not suplhuric acid for the acidification of sodium extract for testing suplhur by lead acetate test?
View SolutionAcetic acid and not sulphuric acid is essential for acidification of sodium extract for testing sulphur by lead acetate test because
View SolutionIt is not advisable to use sulphuric acid in place of acetic acid for acidification while testing sulphur by lead acetate test. Assign reason.
View SolutionFor testing the presence of sulphur by lead acetate test, the Lassaigne's extract is acidified with dilute acetic acid. Can we use dilute hydrochloric or sulphuric acid instead of acetic acid ?
View SolutionAcetic acid and not sulphuric acid is essential for acidification of sodium extract for testing sulphur by lead acetate test because
View Solutionलेड एसीटेट द्वारा सल्फर का परीक्षण करने के लिये सोडियम निष्कर्ष को अम्लीय करने के लिये सल्फ्यूरिक अम्ल के स्थान पर एसिटिक अम्ल का प्रयोग क्यों आवश्यक है?
View Solutionसल्फर के लेड ऐसीटेट द्वारा परीक्षण में सोडियम संगलन निष्कर्ष को ऐसीटेट अम्ल द्वारा उदासीन किया जाता है न H2SO4 कि द्वारा । क्यों ?
View SolutionWhy is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
View SolutionWhy is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
View SolutionWhy is it necessary to use acetic acid and not sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test?
View SolutionWhy is it necessary to use acetic acid and not suplhuric acid for the acidification of sodium extract for testing suplhur by lead acetate test?
View Solution
