(a) What is the role of phosphorous pentoxide in the preparation of N2O5 ?
(b) Phosphine is prepared in an inert atmosphere of CO2. Why ?
( c) Red phosphorous is used for making matches. Why ?
(a) P4O10 acts as strong dehydrating agent. It removes water molecules from nitric acid, HNO3 to form N2O5.
P4O10+12HNO3→4H3PO4+6N2O5.
(b) Phosphine, being inflammable, burns in air. It is therefore prepared in an inert atmosphere.
( c) Red phosphorous is non-poisonous and has a high ignition point and hence red phosphorous is used for making matches.
टॉपर्स ने हल किए ये सवाल
- P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYExercise:Solved Example
Explore 7 Videos
- P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYExercise:Concept Application Exercises 2.1 (Subjective)
Explore 22 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUPBook:CENGAGE CHEMISTRY ENGLISHChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUPExercise:Archives Analytical And Descriptive
Explore 22 Videos
- P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYExercise:Archives Subjective
Explore 10 Videos
समान प्रश्न
(a)What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
(b)Draw the structure of white phosphorus and red phosphorus. Which one of these two types of phosphorus, is more reactive and why ?
View SolutionIn the preparation of P4O6, a mixture of N2andO2 is used rather than pure O2, why ?
View SolutionWhy is not sulphuric acid used for the preparation of CO2 from marble chips?
View SolutionExplain the following.
a. Hydrated barium peroxide is used in the preparation of H2O2 instead of anhydrous barium peroxide.
b. Phosphoric acid is preferred to sulphuric acid in the preparation of H2O2 from barium peroxide..
View SolutionWhy is CO2 made to escape in a lime kiln during the preparation of lime from limestone?
View SolutionThere are two paths for the preparation of phyebnyl-2, 4-dinitro phenyl ether (C). Which path is feasible and why ?
Path I :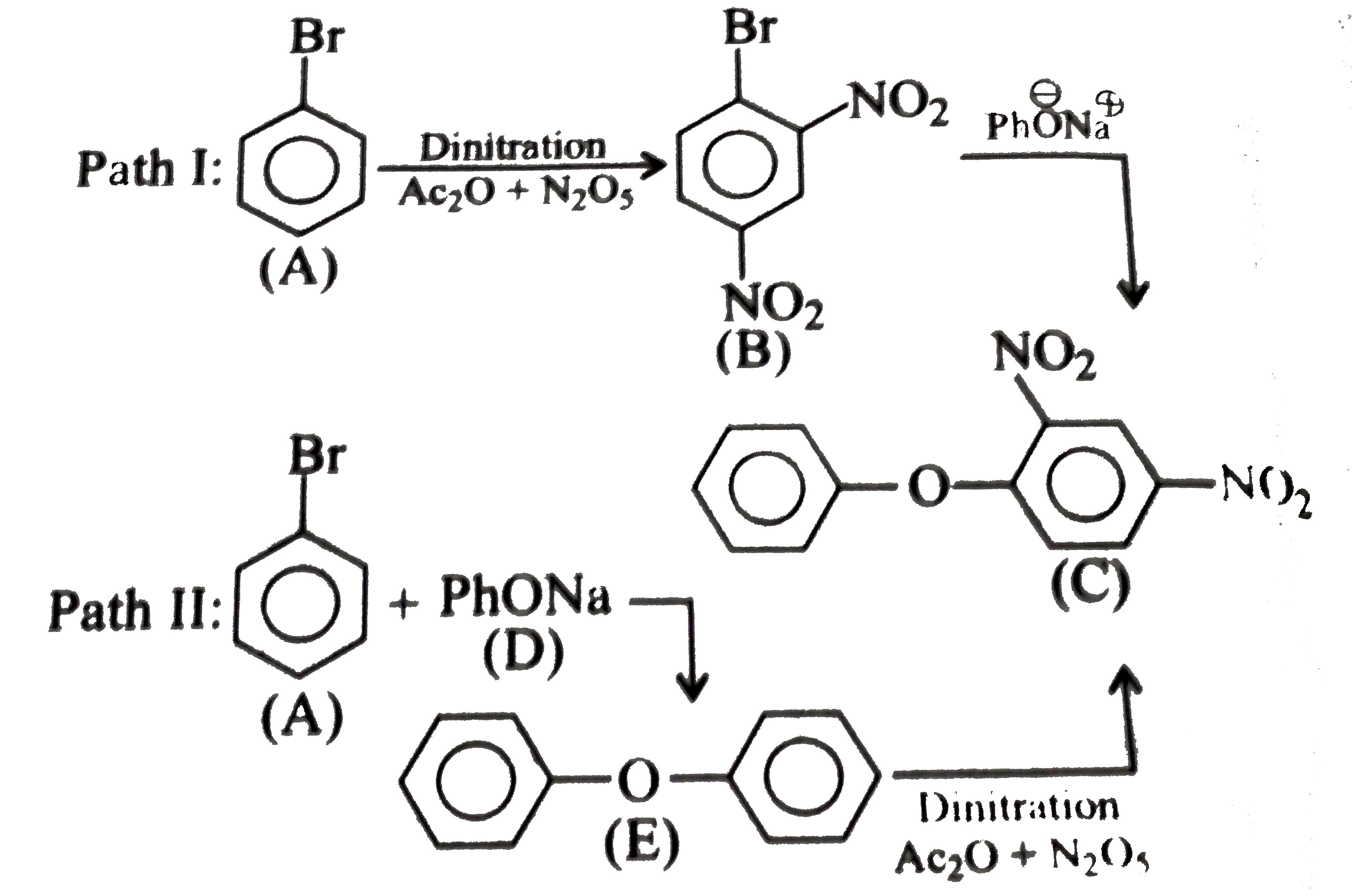
View Solution(a) Why elemental phosphorous does not exist as P2 like N2 ?
(b) NCl3 gets easily hydrolysed, while NF3 does not. Why ?
View SolutionWhat is the proportion of elements present in the following compounds ?
(a) H2O
(b) CO2
(c ) CaO
(d) NO2
View SolutionOutline the preparation of the following compounds using a nucleophilic substitution reaction : CH3CH2−O−N=O
View SolutionBenzene diazonium chloride, C6H5+N2−CI , was decomposed in the presence of hypo phosphorous acid and the nitrogen evolved after drying was found to be 36.9 ml at one atmosphere and 27∘C . The amount of salt taken must be nearly:
View Solution
