The BCl3 is a planar molecule whereas NCl3 is pyramidal because
N−Cl bond is more covalent than B−Cl bond
B−Cl bond is more polar than N−Cl bond
Nitrogen atom is smaller than boron
BCl3 has no lone pair but NCl3 has a lone pair of electron.
The correct Answer is:D
BCl3 is sp3 hybridised hence planar whereas NCl3 is sp3 hybridised with one 1p, hence is pyramidal.
Topper's Solved these Questions
- P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYExercise:Exercises (Integer)
Explore 20 Videos
- P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYExercise:Exercises (Fill In The Blanks)
Explore 15 Videos
- P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 15 ELEMENTS - THE NITROGEN FAMILYExercise:Exercises (Multiple Correct)
Explore 11 Videos
- ORGANIC COMPOUNDS WITH FUNCTIONAL GROUPBook:CENGAGE CHEMISTRY ENGLISHChapter:ORGANIC COMPOUNDS WITH FUNCTIONAL GROUPExercise:Archives Analytical And Descriptive
Explore 22 Videos
- P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYExercise:Archives Subjective
Explore 10 Videos
Similar Questions
Nitrogen forms how many oxides
View SolutionWhen phosphine is bubbled through a solution of silver nitrate ___ is precipitated.
View SolutionWhite phosphorus has.
View SolutionState the equations for the preparation of the following compounds . (Equations need not to be balance).
(i) Chlorobenzene from aniline (in two steps )
(ii) N-Propyl amine from ethyl chloride (in two steps )
View SolutionWater is a liquid, while H2S is a gas at ordinary temperature. Explain.
View SolutionThe BCl_3 is a polar molecule whereas NCl_3 is pyramidal because
View SolutionBCl_3 is a planar molecular because in this molecule boron is
View SolutionBCl3 molecule is planar while NCl3 is pyramidal because
View SolutionThe BF3 is a planar molecule where as NF3 is pyramidal because :
View SolutionThe BCl3 is a planar molecule whereas NCI3 is pyramidal because
View SolutionThe BCl3 is a planar molecule whereas NCI3 is pyramidal because
View SolutionBCl3 अणु समतलीय और NCl3 अणु पिरामिडीय होता है, क्योंकि
View Solutionट्राइमेथिल अमोनिया पिरामिडीय होता है जबकि ट्राइसिलाइल अमोनिया एक समतलीय अनु होता है क्यों?
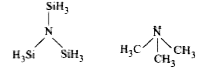
View SolutionThe BCl3 is a planar molecule whereas NCl3 is pyramidal because
View SolutionBCl3 is a planar molecule while NCl3 is pyramidal, because __________.
View SolutionBCl3 is a planar molecule while PCl3 is pyramidal , because________.
View Solution
