Fill in teh blanks.
i. H2SO4+HI→…..+SO2+2H2O
ii. CaOCI2+NaI+HCI→…..+CaCI2+H2O+NaCI
iii. NH3+CI2(excesse)→….+HCI
iv. KMnO4+KCI+H2SO4→K2SO4+MnSO4+…..+…..
v. K2Cr2O7+HCI→KCI+Cr4CI3+.....+....
vi. CuSO4+KI→Cu2I2+.....+....
vii. NH3+NaOCI→N2H4+NaCI+.....
viii. CI2+H2O+HgO→HgCI2.HgO+.....
ix. P4+I2+H2O→H3PO3+.......
x. NaBr+MnO2+H2SO4→NaHSO4+......+H2O+.....
(i) I2, (ii) I2
(iii) NCl3, (iv) Cl2,H2O
(v)H2O,Cl2, (vi) K2SO4,I2
(vii) H2O , (viii) HOCl
(ix)HI, (x)MnSO4,Br2.
Topper's Solved these Questions
- P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYExercise:Exercises (Linked Comprehension)
Explore 26 Videos
- P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYExercise:Exercises (Multiple Correct)
Explore 14 Videos
- P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 17 ELEMENTS - THE HALOGEN FAMILYExercise:Exs 4.1 (Subjective)
Explore 16 Videos
- P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 16 ELEMENTS - THE OXYGEN FAMILYExercise:Archives Subjective
Explore 10 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESExercise:Ex 5.1 (Objective)
Explore 14 Videos
Similar Questions
_S01_475_Q01.png) Conc.H2SO4−−−−−−−→(A)
Conc.H2SO4−−−−−−−→(A)
View Solution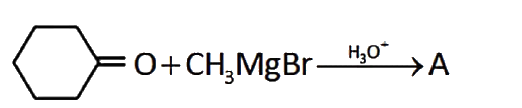
Conc. H2SO4−−−−−−−−→BO3/H2O/Zn−−−−−−−→C. A, B and C are -
View SolutionComplete the following reactions
(a)XeF4+H2O→
(b )XeF6+H2→
(c)XeF6+H2O→
(d)XeF6+NH3→
View SolutionHow much of the following 0.1M solutions are acidic?
a. NH4CI b. NaOH c. HC2H3O2 d. NaCI
e. NH3+NH4CI f. NH3 g. HCI
h. HCIO4 i.(NH4)2SO4 j. K2SO4
View Solution3KClO3+3H2SO4→3KHSO4+HClO4+2ClO2+H2O
Equivalent weight of KClO3 is
View SolutionIdentify X and Y in the given reaction:
P4+H2SO4→X+Y+H2O
View SolutionThe number of reactions that gives SO2 gas :
(a) Cu+conc. H2SO4→
(b) Zn+dil. H2SO4→
(c ) S+H2SO4→r
(d) FeS2+O2→
(e ) NaHSO3+dil. H2SO4→
(f) H2SO4+PCl5→
View SolutionWhat volume of 2 N K2Cr2O7 solution is required to oxidise 0.81 g of H2S in acidic medium. K2Cr2O7+H2S+H2SO4→S+K2SO4+Cr2(SO4)3
View SolutionFeS+KMnO4+H2SO4→Fe2(SO4)3+K2SO4. The equivalent mass FeS in the above reaction is :
View SolutionFor H3PO4 ,
H3PO4→H2PO−4+H+(K1),H2PO−→4HPO−4+H+(K2),
HPO2−4→PO3−4+H+(K3) then
View Solution
