i.(A)NaOH−−−→Δ(B)(g)HCI−−→ While fumes.
ii. After (B) is expelled completely, resultant alkline solution again gives gas (B) on heating with zine
iii.(A) Δ−−→N2O+H2O
What is the formula of white fumes?
NH4NO3
NH4CI
NH4NO2
NH3
The correct Answer is:b
To solve the given problem, we will analyze the steps provided in the question and derive the necessary conclusions step by step.
Step 1: Identify Compound A
The question states that compound A, when heated, produces N2O (dinitrogen oxide) and H2O (water). The only known compound that decomposes to give one mole of N2O and one mole of H2O upon heating is ammonium nitrate (NH4NO3).
Conclusion:
- Compound A is NH4NO3.
Step 2: Reaction of A with NaOH
Next, we consider the reaction of compound A with sodium hydroxide (NaOH). When ammonium nitrate is heated with NaOH, it decomposes to produce ammonia gas (B), sodium nitrate, and water.
The reaction can be summarized as:
NH4NO3+NaOHΔ−→NH3(g)+NaNO3+H2O
Conclusion:
- Gas B is NH3 (ammonia).
Step 3: Reaction of Gas B with HCl
The question states that gas B (ammonia) reacts with hydrochloric acid (HCl) to form white fumes. The reaction between ammonia and hydrochloric acid produces ammonium chloride (NH4Cl), which is a white solid that appears as white fumes when formed in the air.
The reaction can be summarized as:
NH3(g)+HCl(g)→NH4Cl(s)
Conclusion:
- The white fumes are due to the formation of ammonium chloride (NH4Cl).
Step 4: Resultant Alkaline Solution
After gas B (ammonia) is expelled completely, the resultant alkaline solution (which contains sodium nitrate) can also produce gas B (ammonia) upon heating with zinc. This is consistent with the behavior of ammonium compounds in alkaline solutions.
Final Answer
The formula of the white fumes formed is:
NH4Cl
---
The question states that compound A, when heated, produces N2O (dinitrogen oxide) and H2O (water). The only known compound that decomposes to give one mole of N2O and one mole of H2O upon heating is ammonium nitrate (NH4NO3).
- Compound A is NH4NO3.
Next, we consider the reaction of compound A with sodium hydroxide (NaOH). When ammonium nitrate is heated with NaOH, it decomposes to produce ammonia gas (B), sodium nitrate, and water.
NH4NO3+NaOHΔ−→NH3(g)+NaNO3+H2O
- Gas B is NH3 (ammonia).
The question states that gas B (ammonia) reacts with hydrochloric acid (HCl) to form white fumes. The reaction between ammonia and hydrochloric acid produces ammonium chloride (NH4Cl), which is a white solid that appears as white fumes when formed in the air.
NH3(g)+HCl(g)→NH4Cl(s)
- The white fumes are due to the formation of ammonium chloride (NH4Cl).
After gas B (ammonia) is expelled completely, the resultant alkaline solution (which contains sodium nitrate) can also produce gas B (ammonia) upon heating with zinc. This is consistent with the behavior of ammonium compounds in alkaline solutions.
The formula of the white fumes formed is:
NH4Cl
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Multiple Correct) Part-A (Analysis Of Anions)
Explore 30 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Multiple Correct) Part-B (Analysis Of Cations)
Explore 28 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Solved Example
Explore 11 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESExercise:Ex 5.1 (Objective)
Explore 14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 2 Videos
Similar Questions
i.(A)NaOH−−−→Δ(B)(g)HCI−−→ While fumes.
ii. After (B) is expelled completely, resultant alkline solution again gives gas (B) on heating with zine
iii.(A) Δ−−→N2O+H2O
Identify B
View Solutioni.(A)NaOH−−−→Δ(B)(g)HCI−−→ While fumes.
ii. After (B) is expelled completely, resultant alkline solution again gives gas (B) on heating with zine
iii.(A) Δ−−→N2O+H2O
Identify A
View Solution
Knowledge Check
CaC2D2O−−→ARed hot−−−−→Cu tubeB,B is
ATolueneBEthyl-benzeneCBenzeneDButyne
Metal M+airδ−−→AH2O−−→BHCl−−→ White fumes, Metal M can be:
View SolutionMetal M+airδ−−→AH2O−−→BHCl−−→ White fumes, Metal M can be:
View SolutionMetal M+airδ−−→AH2O−−→BHCl−−→ White fumes, Metal M can be:
View Solution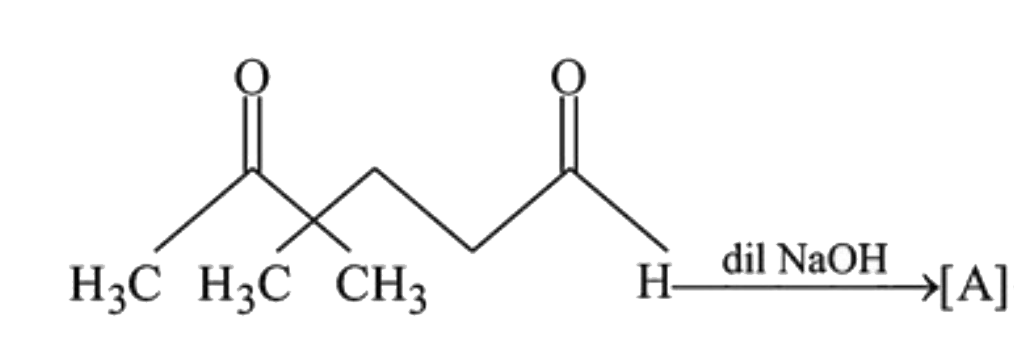 Hdil NaOH−−−−→[A]H2O+−−−→Δ[B]
Hdil NaOH−−−−→[A]H2O+−−−→Δ[B]
View SolutionLi+N2Δ−−→AH2O−−→B+(C) gas
Then the correctly matched pair for A,B,C are :
View SolutionIdentify (A) and (B) in the following:
KO2+SΔ−−→(A)
(A)+BaCI2→BWhite ppt.
View SolutionEtCOCl(A)(i)H2N.OH−−−−−−−→(ii)ӨOH(B)EtOH−−−→(C)
Which of the following statements are correct about the given reaction ?
View Solution
