NH3 solution was added to four semple solution in difference test tube and found the following observation about the precipitate.
a. White ppt which is solution in oxcess of NH3 solution
b.On heating which is white in cold but yellow on heating
c. The cation present in (b) forms white ppt , with hypo solution which give black ppt on heating
d. The cation present in (c ) forms soluble complex with excess of NH3 solution
White ppts in (a),(b) and (c ) respectively obtained are
Zn(OH)2,Zn(OH)2,HgOHg(NH2)NO3
Cd(OH)2,Zn(OH)2,HgOHg(NH2)NO3
HgOHg(NH2)NO3Zn(OH)2,Cd(OH)2
AI(OH)2,Zn(OH)2,Pb(OH)2
The correct Answer is:a,b
To solve the problem step by step, we will analyze each test tube based on the observations provided and identify the compounds responsible for the white precipitates.
Step 1: Analyze Test Tube A
- Observation: White precipitate which is soluble in excess NH₃ solution.
- Analysis: The white precipitate that dissolves in excess NH₃ indicates the presence of either cadmium ions (Cd²⁺) or zinc ions (Zn²⁺).
- Conclusion: The white precipitate in Test Tube A is likely Cadmium Hydroxide (Cd(OH)₂) or Zinc Hydroxide (Zn(OH)₂).
Step 2: Analyze Test Tube B
- Observation: White precipitate that is white in cold but yellow on heating.
- Analysis: This behavior is characteristic of zinc hydroxide (Zn(OH)₂), which is white when cold and turns yellow when heated due to the formation of zinc oxide (ZnO).
- Conclusion: The white precipitate in Test Tube B is Zinc Hydroxide (Zn(OH)₂).
Step 3: Analyze Test Tube C
- Observation: The cation present forms a white precipitate with hypo solution, which gives a black precipitate on heating.
- Analysis: The cation that fits this description is mercuric ion (Hg²⁺), which forms a white precipitate of mercuric hydroxide (Hg(OH)₂) with NH₃. When treated with hypo (thiosulfate), it forms mercuric thiosulfate (Hg₂S₂O₃), which on heating gives a black precipitate of mercuric sulfide (HgS).
- Conclusion: The white precipitate in Test Tube C is Mercuric Hydroxide (Hg(OH)₂).
Final Summary of White Precipitates
- Test Tube A: Cadmium Hydroxide (Cd(OH)₂) or Zinc Hydroxide (Zn(OH)₂)
- Test Tube B: Zinc Hydroxide (Zn(OH)₂)
- Test Tube C: Mercuric Hydroxide (Hg(OH)₂)
- Observation: White precipitate which is soluble in excess NH₃ solution.
- Analysis: The white precipitate that dissolves in excess NH₃ indicates the presence of either cadmium ions (Cd²⁺) or zinc ions (Zn²⁺).
- Conclusion: The white precipitate in Test Tube A is likely Cadmium Hydroxide (Cd(OH)₂) or Zinc Hydroxide (Zn(OH)₂).
- Observation: White precipitate that is white in cold but yellow on heating.
- Analysis: This behavior is characteristic of zinc hydroxide (Zn(OH)₂), which is white when cold and turns yellow when heated due to the formation of zinc oxide (ZnO).
- Conclusion: The white precipitate in Test Tube B is Zinc Hydroxide (Zn(OH)₂).
- Observation: The cation present forms a white precipitate with hypo solution, which gives a black precipitate on heating.
- Analysis: The cation that fits this description is mercuric ion (Hg²⁺), which forms a white precipitate of mercuric hydroxide (Hg(OH)₂) with NH₃. When treated with hypo (thiosulfate), it forms mercuric thiosulfate (Hg₂S₂O₃), which on heating gives a black precipitate of mercuric sulfide (HgS).
- Conclusion: The white precipitate in Test Tube C is Mercuric Hydroxide (Hg(OH)₂).
- Test Tube A: Cadmium Hydroxide (Cd(OH)₂) or Zinc Hydroxide (Zn(OH)₂)
- Test Tube B: Zinc Hydroxide (Zn(OH)₂)
- Test Tube C: Mercuric Hydroxide (Hg(OH)₂)
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Multiple Correct) Part-A (Analysis Of Anions)
Explore 30 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Exercises (Multiple Correct) Part-B (Analysis Of Cations)
Explore 28 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Solved Example
Explore 11 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESExercise:Ex 5.1 (Objective)
Explore 14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:SUBJECTIVE TYPE
Explore 2 Videos
Similar Questions
NH3 solution was added to four semple solution in difference test tube and found the following observation about the precipitate.
a. White ppt which is solution in oxcess of NH3 solution
b.On heating which is white in cold but yellow on heating
c. The cation present in (b) forms white ppt , with hypo solution which give black ppt on heating
d. The cation present in (c ) forms soluble complex with excess of NH3 solution
White ppt in (c) and the soluble complex from white ppt with the type solution is//are
View SolutionWhich of the following ppt is insoluble in NH3 solution ?
View Solution
Knowledge Check
Which of the following will not give white precipitate with
ammoniacal silver nitrate solution ?ACH3−C≡C−CH3B C
C D
D
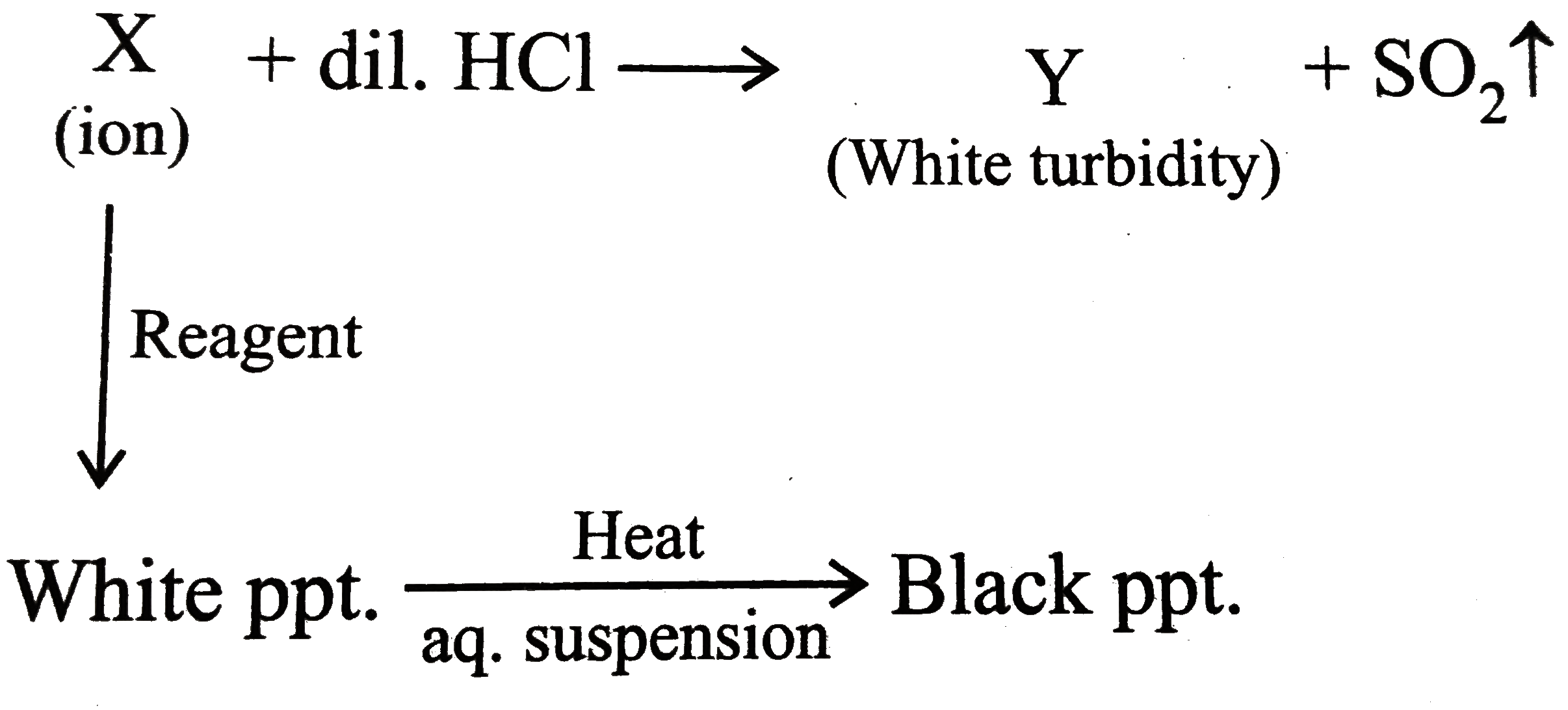
Which of the following cation may be present in white ppt ?
View SolutionWhich of the following nitrate salt solution neither produce ppt. with excess NaOH nor with excess NH4OH solution?
View SolutionWhite ppt of PbCl2 is soluble in aq NH3. (T/F)
View SolutionWhich of the following will not form a yellow precipitate on heating with an alkaline solution of iodine?
View SolutionIdentify the cations in each of the following case :
NaOH Solution when added to Solution (C) gives white ppt which is insoluble in excess.
View SolutionIdentify the cations in each of the following case :
NH4OH Solution when added to the Solution (B) gives white ppt which does not dissolve in excess.
View SolutionThe aqueous solution of salt gives white ppt with lead acetate solution which is insoluble in hot water and nitric acid .The salt contains
View Solution
