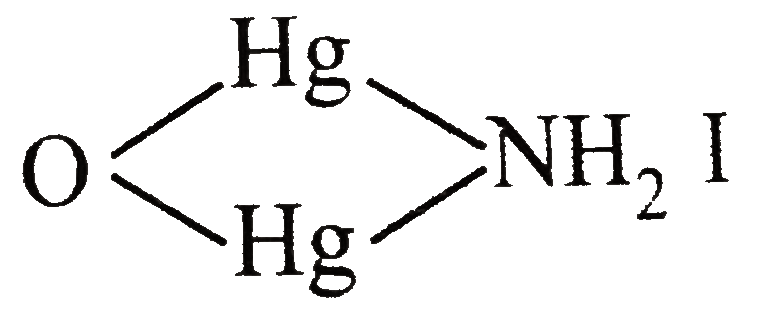
HgCI2+excess ofKI→(A)−−→NH3(B) ,(A) and (B) respectively are
(Y),(X)
both (X)
both (Y)
The correct Answer is:a
To solve the question, we need to determine the compounds A and B formed from the reaction of mercury(II) chloride (HgCl₂) with excess potassium iodide (KI) and then with ammonia (NH₃) and sodium hydroxide (NaOH).
1. Reaction of HgCl₂ with KI:
- When mercury(II) chloride (HgCl₂) reacts with excess potassium iodide (KI), it undergoes a double displacement reaction.
- The reaction can be represented as:
HgCl2+2KI→HgI2+2KCl
- Here, mercuric iodide (HgI₂) is formed, which is a scarlet precipitate. Thus, we identify A as:
A=HgI2(scarlet precipitate of mercuric iodide)
2. Further Reaction of HgI₂ with NH₃ and NaOH:
- The next step involves treating the mercuric iodide (HgI₂) with ammonia (NH₃) and sodium hydroxide (NaOH).
- The reaction can be represented as:
HgI2+2KI+2NH3+2NaOH→K2HgI4+2NaCl+2NH4I
- The product formed in this reaction is potassium tetraiodomercurate(II) (K₂HgI₄), which is also known as Nessler's reagent. Thus, we identify B as:
B=K2HgI4(Nessler's reagent)
Final Answer:
- A = HgI₂ (scarlet precipitate of mercuric iodide)
- B = K₂HgI₄ (Nessler's reagent)
- When mercury(II) chloride (HgCl₂) reacts with excess potassium iodide (KI), it undergoes a double displacement reaction.
- The reaction can be represented as:
HgCl2+2KI→HgI2+2KCl
- Here, mercuric iodide (HgI₂) is formed, which is a scarlet precipitate. Thus, we identify A as:
A=HgI2(scarlet precipitate of mercuric iodide)
- The next step involves treating the mercuric iodide (HgI₂) with ammonia (NH₃) and sodium hydroxide (NaOH).
- The reaction can be represented as:
HgI2+2KI+2NH3+2NaOH→K2HgI4+2NaCl+2NH4I
- The product formed in this reaction is potassium tetraiodomercurate(II) (K₂HgI₄), which is also known as Nessler's reagent. Thus, we identify B as:
B=K2HgI4(Nessler's reagent)
- A = HgI₂ (scarlet precipitate of mercuric iodide)
- B = K₂HgI₄ (Nessler's reagent)
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Viva Voce Questions And Part-A (Analysis Of Anions)
30 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Viva Voce Questions And Part-B (Dry Tests)
9 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Ex 8.1
14 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESEXERCISE - Ex 5.1 (Objective)
14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSEXERCISE - SUBJECTIVE TYPE
2 Videos
Similar Questions
Knowledge Check
RMgX(i)CH3CN−−−−−−→(ii)NH4Cl(A)RMgX−−−→NH4Cl(B), (B) will be :
A1∘ROHB2∘ROHC3∘ROHDAlkene
KO2+CO2+H2Omore−−−−−→CO2A+B , then A and B are respectively
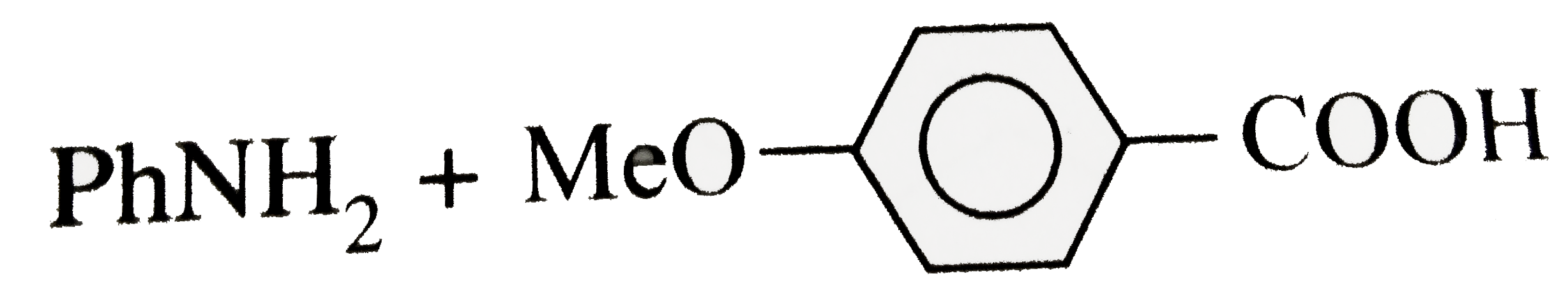
View SolutionAldehyde (A) NH2OH.HCl−−−−−−−−→ (B) H⊕−−→MeNH2+HCOOH
Aldehyde (A) and compound (B), respectively are:
Aldehyde (A)
I. MeCHO II. HCHO
Compound (B)
III.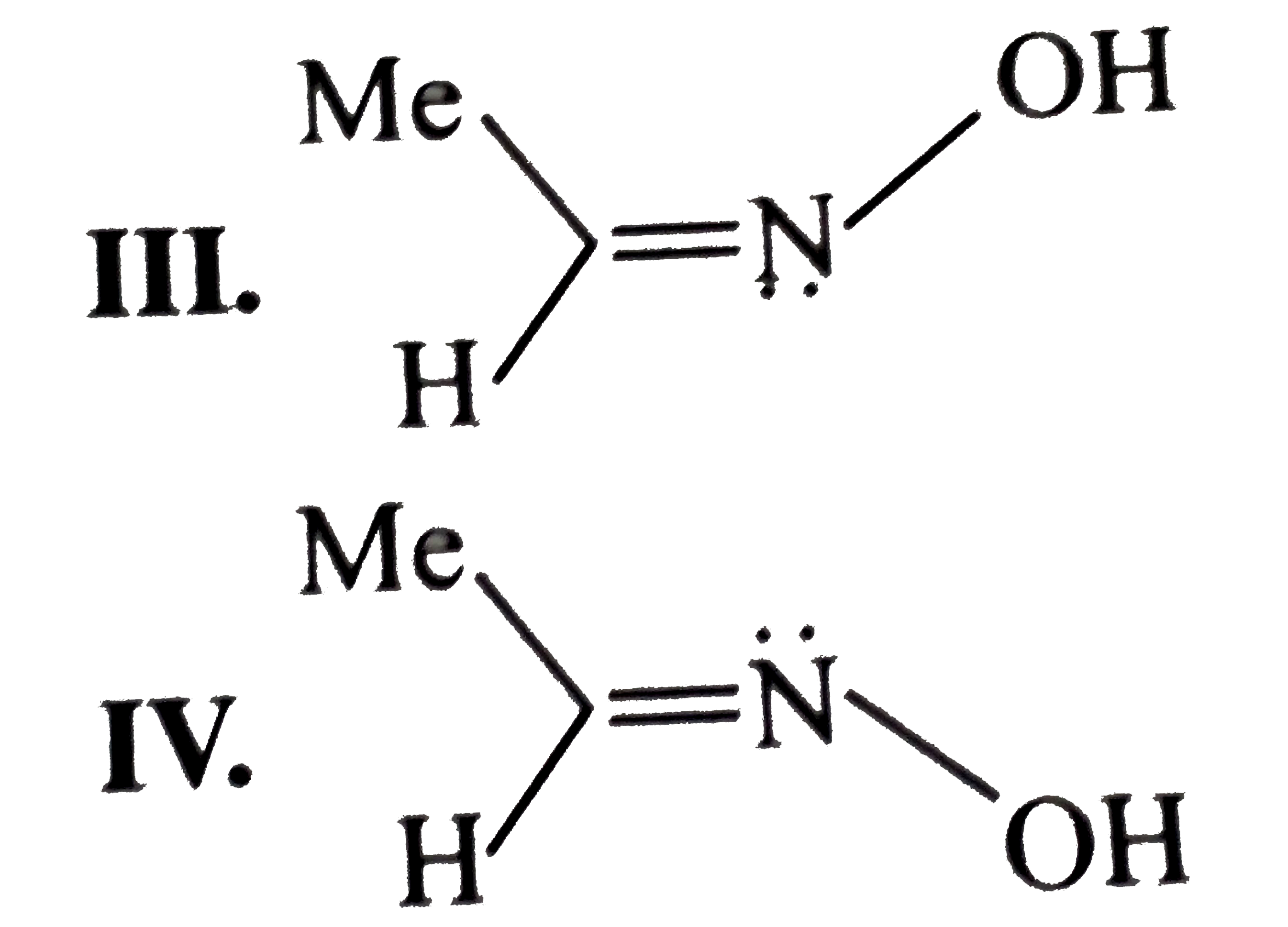
View SolutionIn the chemical reaction ,
CH3CH2NH2+CHCl3+3KOH→(A)+(B)+3H2O
The compounds (A) and (B) are respectively:
View SolutionCl2+NH3 (excess) →A+B
View SolutionCH3−CH2−C∣∣O−NH2A−−→CH3−CH2−NH2
B−−→CH3−CH2−OH
In the above sequence A & B respectively are-
View SolutionCH3CH2NH2(CH3CO)2−−−−−−→ΔA+B, (A) and (B) are
View SolutionCH3CH2NH2(CH3CO)2O−−−−−−−→ΔA+B, (A) and (B) are
View Solution