Given salt is a bromide or iodide. How will you identify it by treating the salt with chlorine water and CS2?
Video Solution
Text Solution
If CS2 layer assumes an orange colour, it is Brɵ if CS2 layer assumes a violet colour it is Iɵ
|
Topper's Solved these Questions
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Viva Voce Questions And Part-B (Dry Tests)
9 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Viva Voce Questions And Part-C (Analysis Of Cations)
42 Videos
- QUALITATIVE INORGANIC SALT ANALYSISBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - QUALITATIVE INORGANIC SALT ANALYSISEXERCISE - Ex 8.2
78 Videos
- P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - P-BLOCK GROUP 18 ELEMENTS - THE INERT GASESEXERCISE - Ex 5.1 (Objective)
14 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSEXERCISE - SUBJECTIVE TYPE
2 Videos
Similar Questions
Water of often treated with chlorine to
View SolutionWater of often treated with chlorine to
View SolutionWhat happens when ethene is treated with chlorine water
View Solution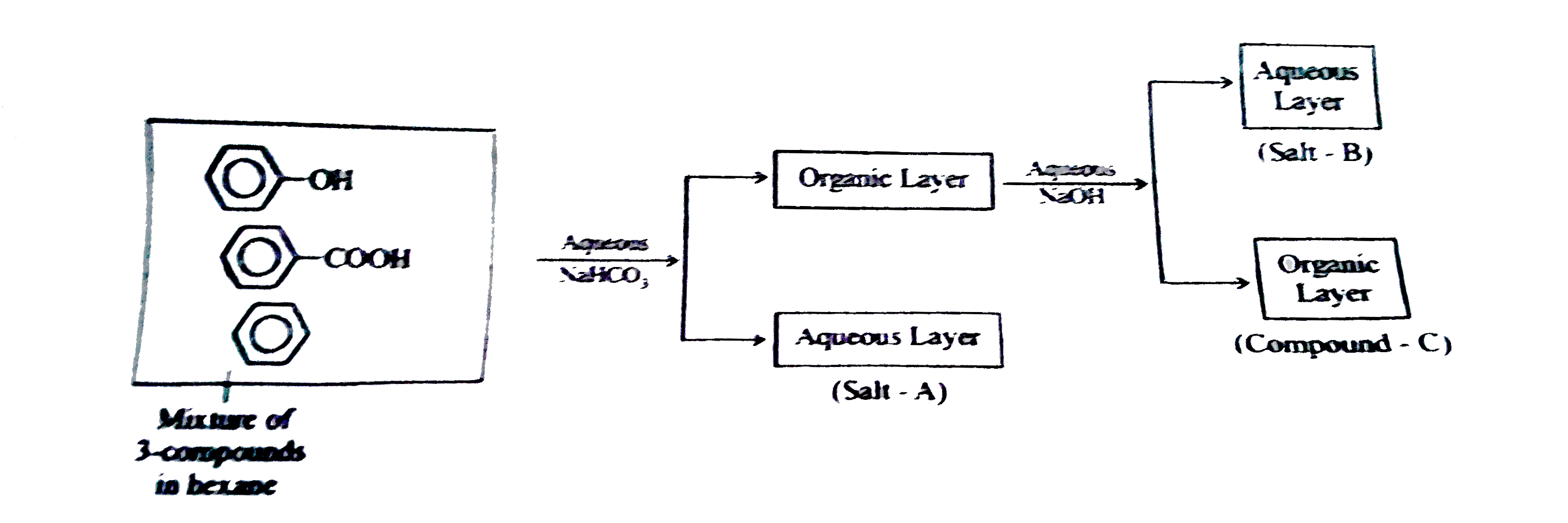
Q. identify salt 'A'?
View SolutionHow will you convert ethyl bromide into ethane.
View SolutionThe salt having the least solubility in water is :
View SolutionThe salt having the least solubility in water is :
View SolutionHow will you convert ethyl bromide into ethoxyethane.
View SolutionHow will you convert ethyl bromide into butane.
View SolutionYou are provided the solutions of sodium hydroxide (A), hydrochloric acid (B), sodium chloride ( C) and phenolphthalein (P) separately. All the solutions are colourless. How will you identify the solutions of acid, base and salt?
View Solution

