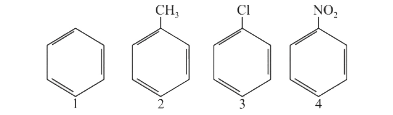
Identify the correct of reactivity in electrophilic substitution reaction of the following compounds.
(1) Benzene
(2) Toluene
(3) Chlorobenzene,
(4) Nitrobenzene.
(1)>(2)>(3)>(4)
(4)>(3)>(2)>(1)
(2)>(1)>(3)>(4)
(2)>(3)>(1)>(4)
The correct Answer is:C
To determine the correct order of reactivity in electrophilic substitution reactions for the given compounds: benzene, toluene, chlorobenzene, and nitrobenzene, we need to analyze the effects of substituents on the benzene ring.
1. Understanding Electrophilic Substitution:
- Electrophilic substitution reactions involve an electrophile attacking the benzene ring, resulting in the substitution of a hydrogen atom. The reactivity of the benzene ring in these reactions is influenced by the nature of substituents attached to it.
2. Analyzing Benzene:
- Benzene (C6H6) has no substituents. It is a symmetrical compound and has a moderate reactivity towards electrophilic substitution.
3. Analyzing Toluene:
- Toluene (C6H5CH3) has a methyl group (–CH3) attached to the benzene ring. The methyl group is an electron-donating group due to its +I (inductive) effect and hyperconjugation. This increases the electron density on the benzene ring, making it more reactive towards electrophilic substitution compared to benzene.
4. Analyzing Chlorobenzene:
- Chlorobenzene (C6H5Cl) has a chlorine atom attached to the benzene ring. Chlorine exhibits a –I (inductive) effect, which withdraws electron density from the ring, decreasing its reactivity. However, chlorine also has a +R (resonance) effect, which can stabilize the ring and direct substitution to the ortho and para positions. Overall, the –I effect makes chlorobenzene less reactive than toluene but more reactive than nitrobenzene.
5. Analyzing Nitrobenzene:
- Nitrobenzene (C6H5NO2) has a nitro group (–NO2) attached to the benzene ring. The nitro group is a strong –I (inductive) and –R (resonance) withdrawing group, which significantly decreases the electron density on the benzene ring. This makes nitrobenzene the least reactive towards electrophilic substitution.
6. Establishing the Order of Reactivity:
- Based on the analysis:
- Toluene (most reactive) > Benzene > Chlorobenzene > Nitrobenzene (least reactive).
- Therefore, the correct order of reactivity in electrophilic substitution reactions is:
- Toluene > Benzene > Chlorobenzene > Nitrobenzene.
Final Answer:
The correct order of reactivity in electrophilic substitution reaction is:
Toluene > Benzene > Chlorobenzene > Nitrobenzene.
- Electrophilic substitution reactions involve an electrophile attacking the benzene ring, resulting in the substitution of a hydrogen atom. The reactivity of the benzene ring in these reactions is influenced by the nature of substituents attached to it.
- Benzene (C6H6) has no substituents. It is a symmetrical compound and has a moderate reactivity towards electrophilic substitution.
- Toluene (C6H5CH3) has a methyl group (–CH3) attached to the benzene ring. The methyl group is an electron-donating group due to its +I (inductive) effect and hyperconjugation. This increases the electron density on the benzene ring, making it more reactive towards electrophilic substitution compared to benzene.
- Chlorobenzene (C6H5Cl) has a chlorine atom attached to the benzene ring. Chlorine exhibits a –I (inductive) effect, which withdraws electron density from the ring, decreasing its reactivity. However, chlorine also has a +R (resonance) effect, which can stabilize the ring and direct substitution to the ortho and para positions. Overall, the –I effect makes chlorobenzene less reactive than toluene but more reactive than nitrobenzene.
- Nitrobenzene (C6H5NO2) has a nitro group (–NO2) attached to the benzene ring. The nitro group is a strong –I (inductive) and –R (resonance) withdrawing group, which significantly decreases the electron density on the benzene ring. This makes nitrobenzene the least reactive towards electrophilic substitution.
- Based on the analysis:
- Toluene (most reactive) > Benzene > Chlorobenzene > Nitrobenzene (least reactive).
- Therefore, the correct order of reactivity in electrophilic substitution reactions is:
- Toluene > Benzene > Chlorobenzene > Nitrobenzene.
The correct order of reactivity in electrophilic substitution reaction is:
Toluene > Benzene > Chlorobenzene > Nitrobenzene.
Topper's Solved these Questions
- ORGANIC REACTION MECHANISMBook:CENGAGE CHEMISTRY ENGLISHChapter:ORGANIC REACTION MECHANISMExercise:True/False
Explore 2 Videos
- ORGANIC REACTION MECHANISMBook:CENGAGE CHEMISTRY ENGLISHChapter:ORGANIC REACTION MECHANISMExercise:Analytical and Descriptive
Explore 6 Videos
- ORGANIC REACTION MECHANISMBook:CENGAGE CHEMISTRY ENGLISHChapter:ORGANIC REACTION MECHANISMExercise:Assertion-Reasoning
Explore 12 Videos
- NCERT BASED EXERCISEBook:CENGAGE CHEMISTRY ENGLISHChapter:NCERT BASED EXERCISEExercise:Chemical Equilibrium
Explore 73 Videos
- P-BLOCK GROUP 13 - BORON FAMILYBook:CENGAGE CHEMISTRY ENGLISHChapter:P-BLOCK GROUP 13 - BORON FAMILYExercise:Exercise Archives (Subjecive)
Explore 9 Videos
Similar Questions
Knowledge Check
The correct order of reactivity towards electrophilic substitution is
Abenzene > phenol > benzoic acid > chlorobenzeneBphenol > benzene > chlorobenzene > benzoic acidCchlorobenzene > benzoic acid > phenol > benzeneDbenzoic acid > chlorobenzene > benzenegtphenol.
Increasing order of electrophilic substitution for following compounds:
View SolutionWhich of the following is most reactive for electrophilic substitution reaction
View SolutionToluene o/p orienting with respect to an electrophilic substitution reaction due to
View SolutionThe most deactivating group for electrophilic substitution reaction in benzene ring is
View SolutionIncreasing order of the following for electrophilic substitution reaction as -
_E01_194_Q01.png)
View SolutionThe correct order of reactivity towards the electrophilic substitiution of the compounds aniline(I),benzene(II) and nitro-benzene(III) is
View SolutionFor an electrophilic substitution reaction , the presence of a halogen atom in the benzene ring …..
View Solution