Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Due to strong -R and -I effect of the (−−−NO2) group, electron density in the (O−−−H) bond decreases and hence the loss of a proton becomes easy.
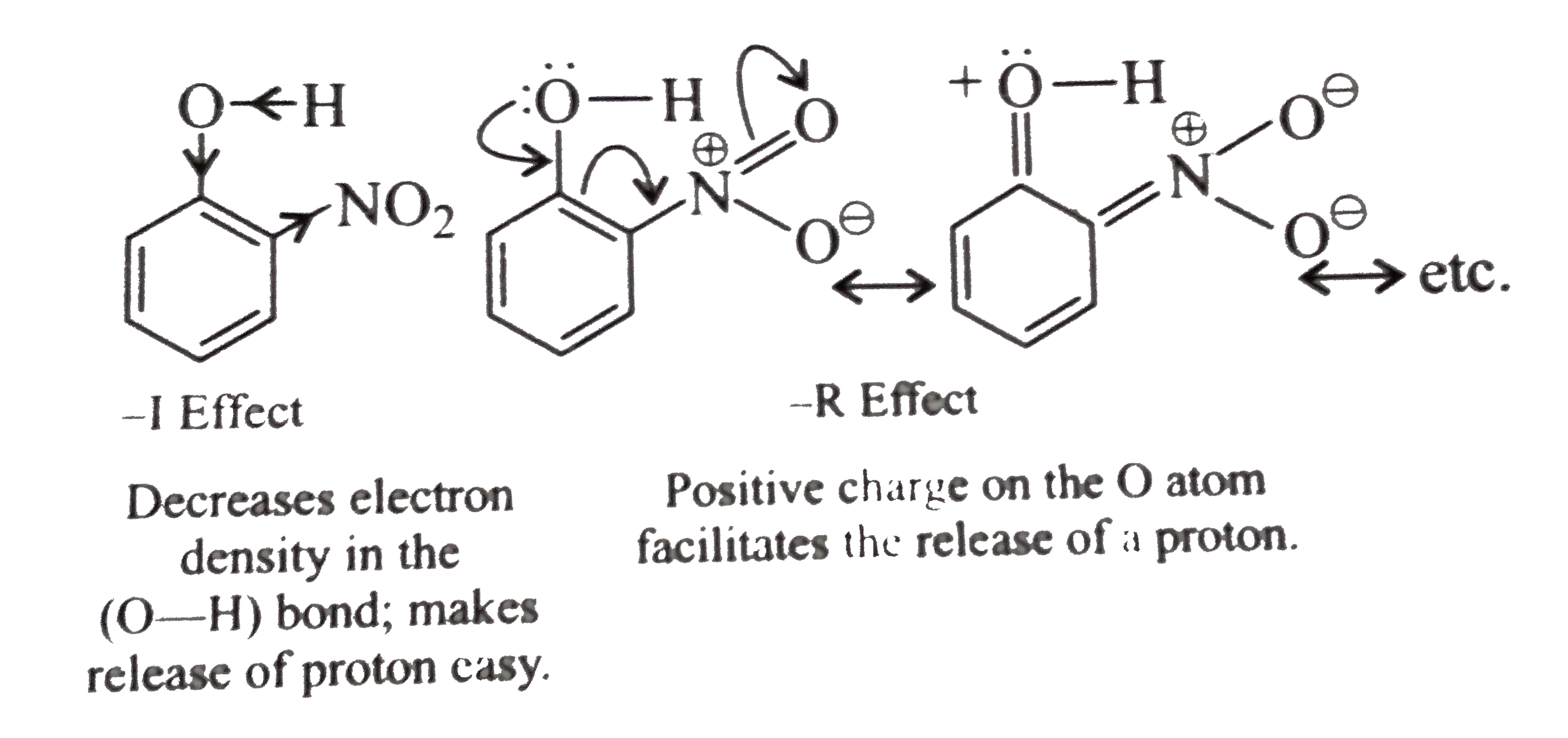
Further, after the loss of a proton, the o-nitrophenoxide ion left behind is stabilised by reasonance.
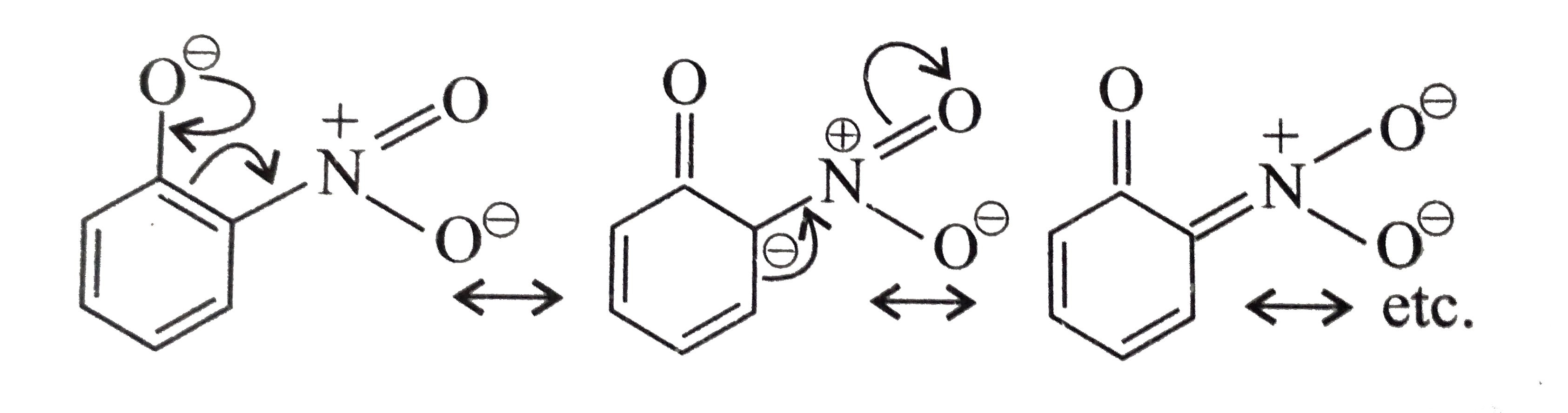
o-Nitrophenoxide ion is stabilised by resonance, thereby making o-nitrophenol a stronger acid. In contrast, due to +R effect to the (OCH3) group, it increases the electron-density in the (O−−−H) bond, thereby making the loss of proton difficult.
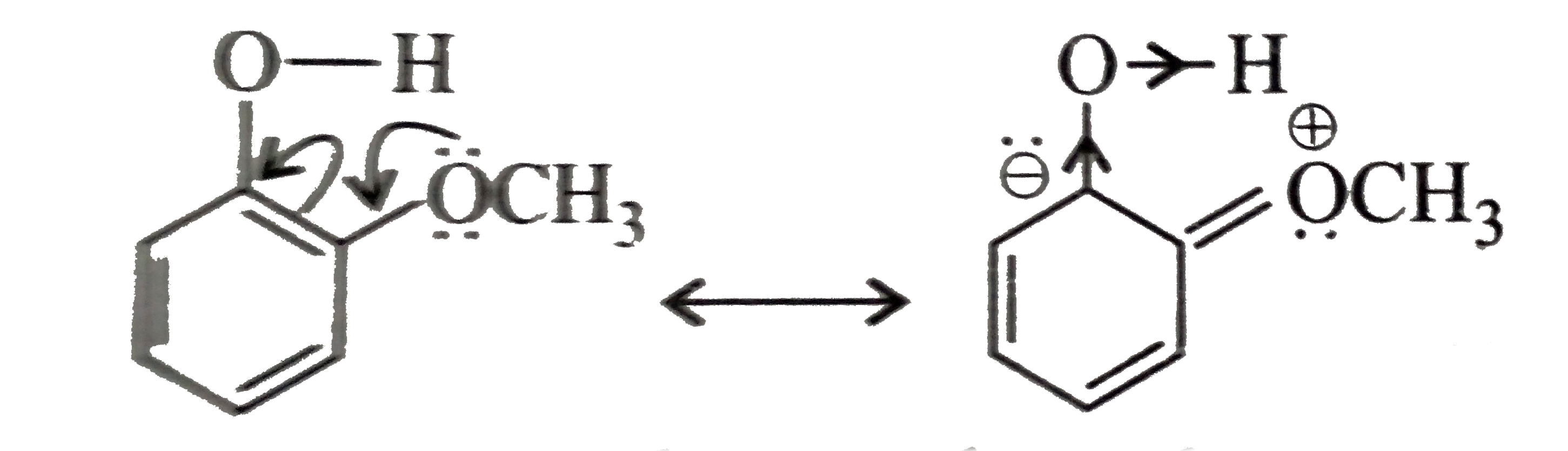
Furthermore, the o-methoxyphenoxide ion left after the loss of a proton is destabilised by resonance.
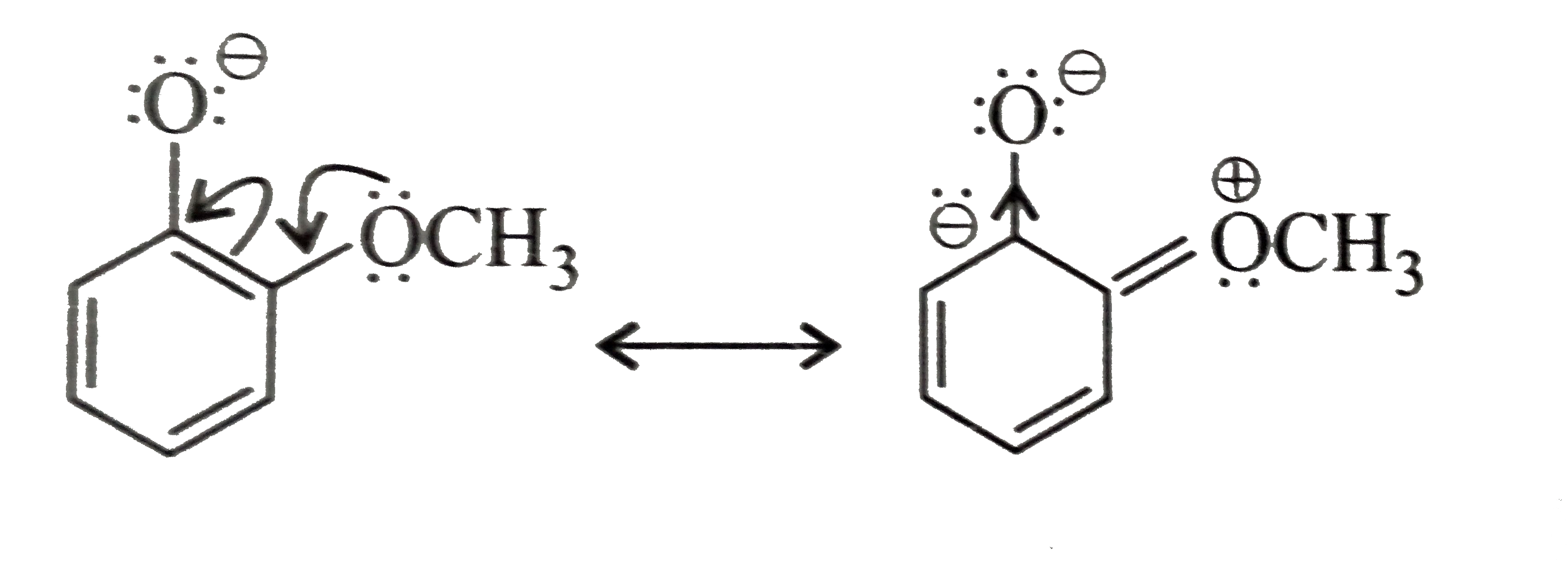
The two negative charges repel each other, thereby destabilising the o-methoxyphenoxide ion. Thus, o-nitropenol is more acidic than o-methoxy-phenol.
Topper's Solved these Questions
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Exercises Linked Comprehension
Explore 53 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Exercises Multiple Correct
Explore 17 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Exercises Subjective
Explore 23 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRY ENGLISHChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Archives Subjective
Explore 26 Videos
Similar Questions
Phenol is more acidic than :
View SolutionExplain why p-nitrophenol is more acidic than phenol ?
View SolutionStatement-1: Ortho nitro phenol is more acidic than orthomethoxy Phenol
Statement-2: NO2 is a deactivating group while OCH3 is activating group .
View SolutionMore acidic than ethanol is
View SolutionAssertion : Ortho nitro phenol is more acidic than meta nitro phenol
Reason : Ortho nitro phenol has more –I effect than meta nitro phenol
View SolutionExplain the following observations:
o-and p-nitrophenols are more acidic than phenol.
View SolutionExplain the following observations:
Phenol is more acidic than ethanol.
View SolutionFormic acid is more acidic than
View SolutionWhy is acetic acid more acidic than ethanol?
View Solutiono-nitrophenol is more volatile than p-nitrophenol it is due to
View Solution
