A 0.535 gm mixture of ethanol and acetaldehyde when heated with Fehling's solution gave 1.2 gm of a red precipitate. What is the percentage of acetaldethyde in the mixture ?
Let three be x gm of CH3CHO and (0.535−xgm) of C2H5OH.
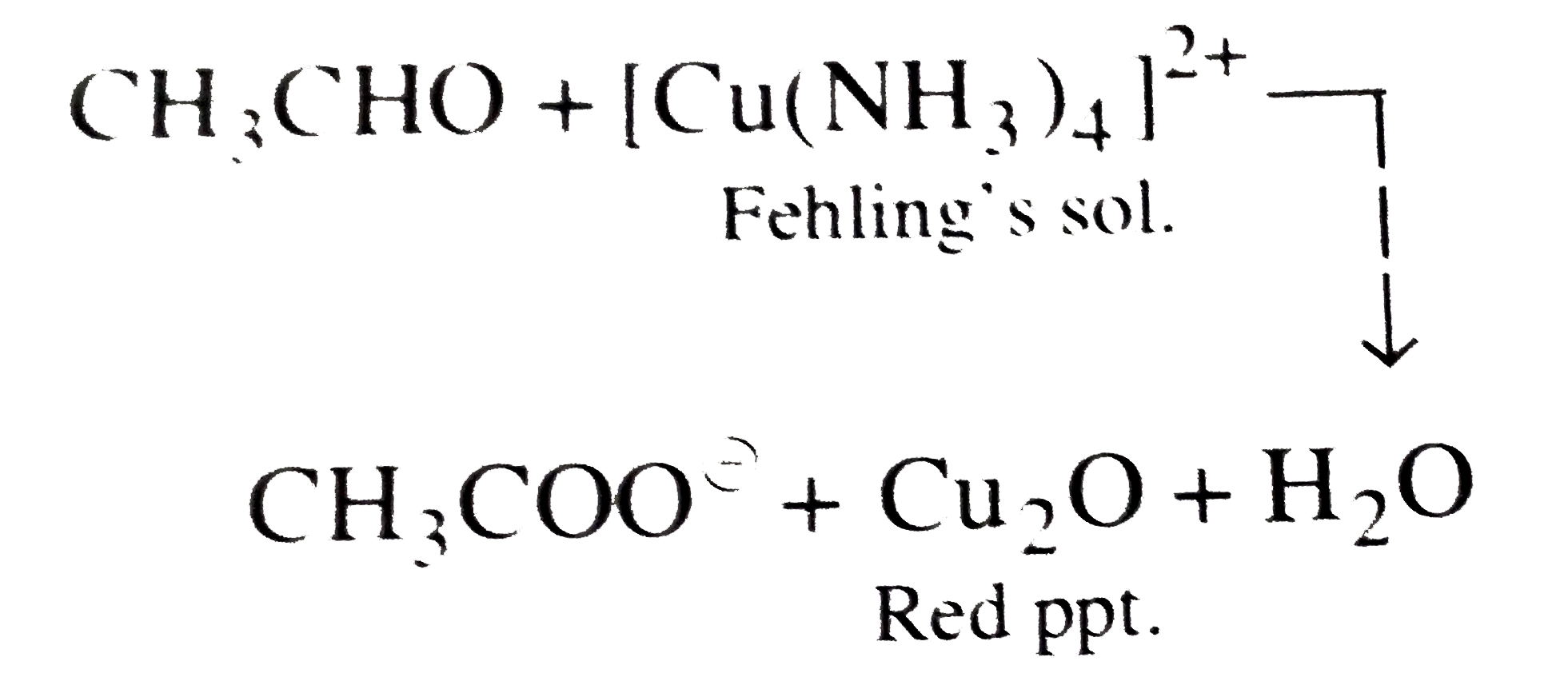
(Only aldehyde reacts with Fehiling's solution, not alcohol)
1 mol of CH3CHO=1(44gm) mol of red ppt. of Cu2O(143gm)
143 gm of precipitate is obtained from 44 gm of CH3CHO. 1.2 gm of precipitate is obtained from:
⇒4143×1.2≈0.348 gm of CH3CHO
% of CH3CHO=0.3680.535×100≈68.8%
Topper's Solved these Questions
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESEXERCISE - Exercises Concept Application
20 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESEXERCISE - Exercises Linked Comprehension
29 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESEXERCISE - Solved Examples
10 Videos
- ALCOHOL,PHENOL AND ETHERSBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - ALCOHOL,PHENOL AND ETHERSEXERCISE - Archives Analytical And Descriptive
14 Videos
- APPENDIX INORGANIC VOLUME 2BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - APPENDIX INORGANIC VOLUME 2EXERCISE - Short Answer Type
178 Videos
Similar Questions
Knowledge Check
When acetaldehyde is heated with Fehling solution, it gives a precipitate of
ACuBCuOCCu2ODCu+CuO+Cu2OWhen acetaldehyde is heated with Fehling solution, it gives a precipitate of
ACuBCuOCCu2ODCu+CuO+Cu2OWhen acetaldehyde is heated with Fehling solution, it gives a precipitate of
ACuBCuOCCu2ODCu+CuO+Cu2O
12 Litres of CO2 gas is obtained on complete combustion of 5 litre gaseous mixture of ehtane and propane then what will be the percentage amount of ethane in mixture ?
View SolutionNeutralisation of 30 gm of a mixture of acetic acid and phenol solutions required 100 ml of 2M sodium hydroxide solution. When the same mixture was treated with bromine water, 33.1 gm of precipitate was formed. Determine the mass percentage of acetic acid and phenol in the given solution.
View SolutionAn aqueous solution of a mixture of two inorganic salts when treated with dilute HCl, gave a precipitate (P) and a filtrate (Q). The precipitate (P) was found to dissolve in hot water. The filtrate (Q) remained unchanged, when treated with H2S in a dilute mineral acid medium. However, it gave a green precipitate (R) in with H2S in ammoniacal medium. The precipitate (R) gave a coloured solution (S), when treated with H2O2 in an aqueous NaOH medium.
The coloured solution (S) contains
View SolutionAn aqueous solution of a mixture of two inorganic salts when treated with dilute HCl, gave a precipitate (P) and a filtrate (Q). The precipitate (P) was found to dissolve in hot water. The filtrate (Q) remained unchanged, when treated with H2S in a dilute mineral acid medium. However, it gave a green precipitate (R) H2S in an ammoniacal medium. The precipitate (R) gave a coloured solution (S), when treated with H2O2 in an aqueous NaOH medium.
The precipitate P contains
View SolutionWhen 15 g of a mixture of NaCl and Na2CO3 is heated with dilute HCl, 2.5 g of CO3 is evolved at NTP. Calculate percentage composition of the original mixture.
View Solution

