Among the following, the best and the least HΘ ion donor, respectively, are:
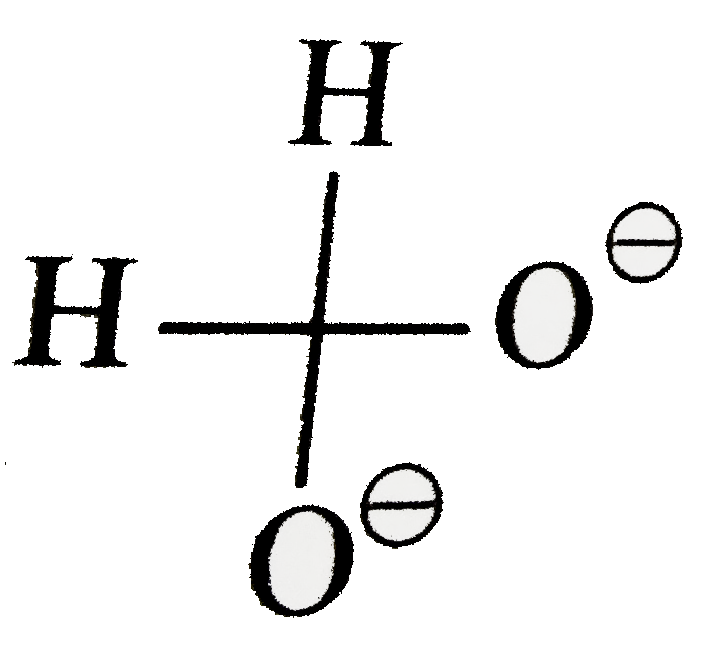
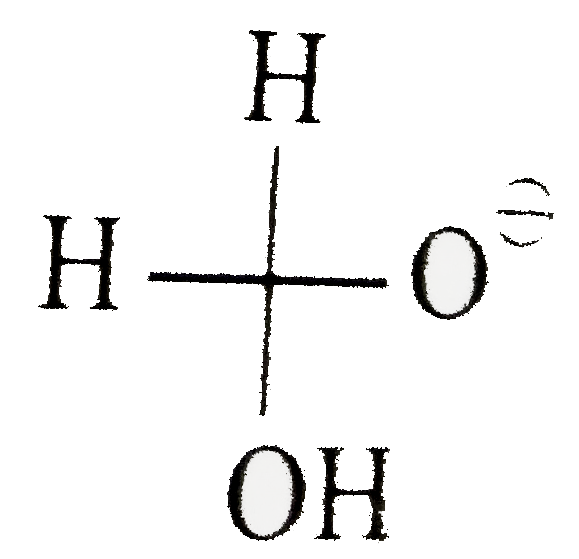
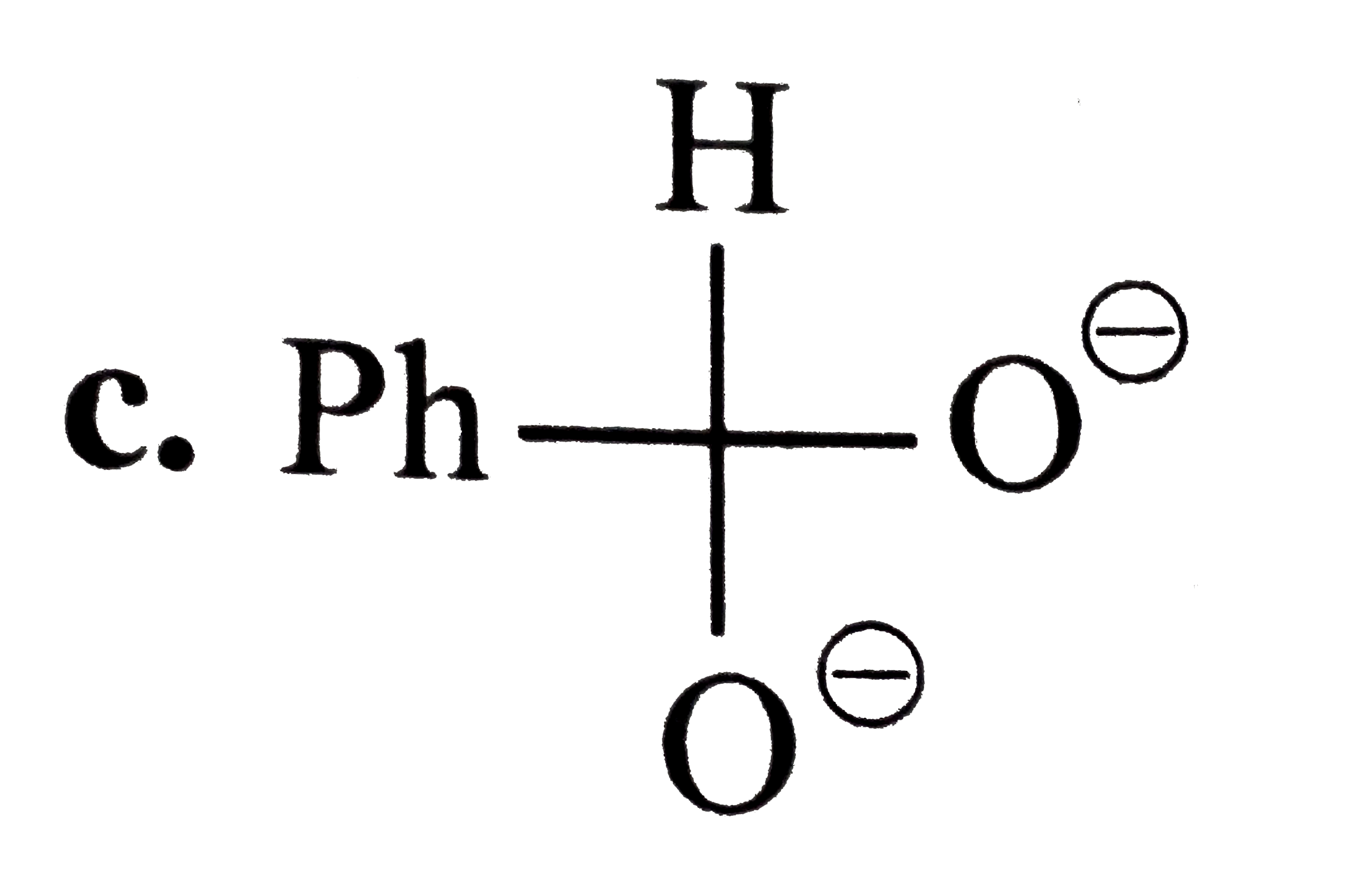
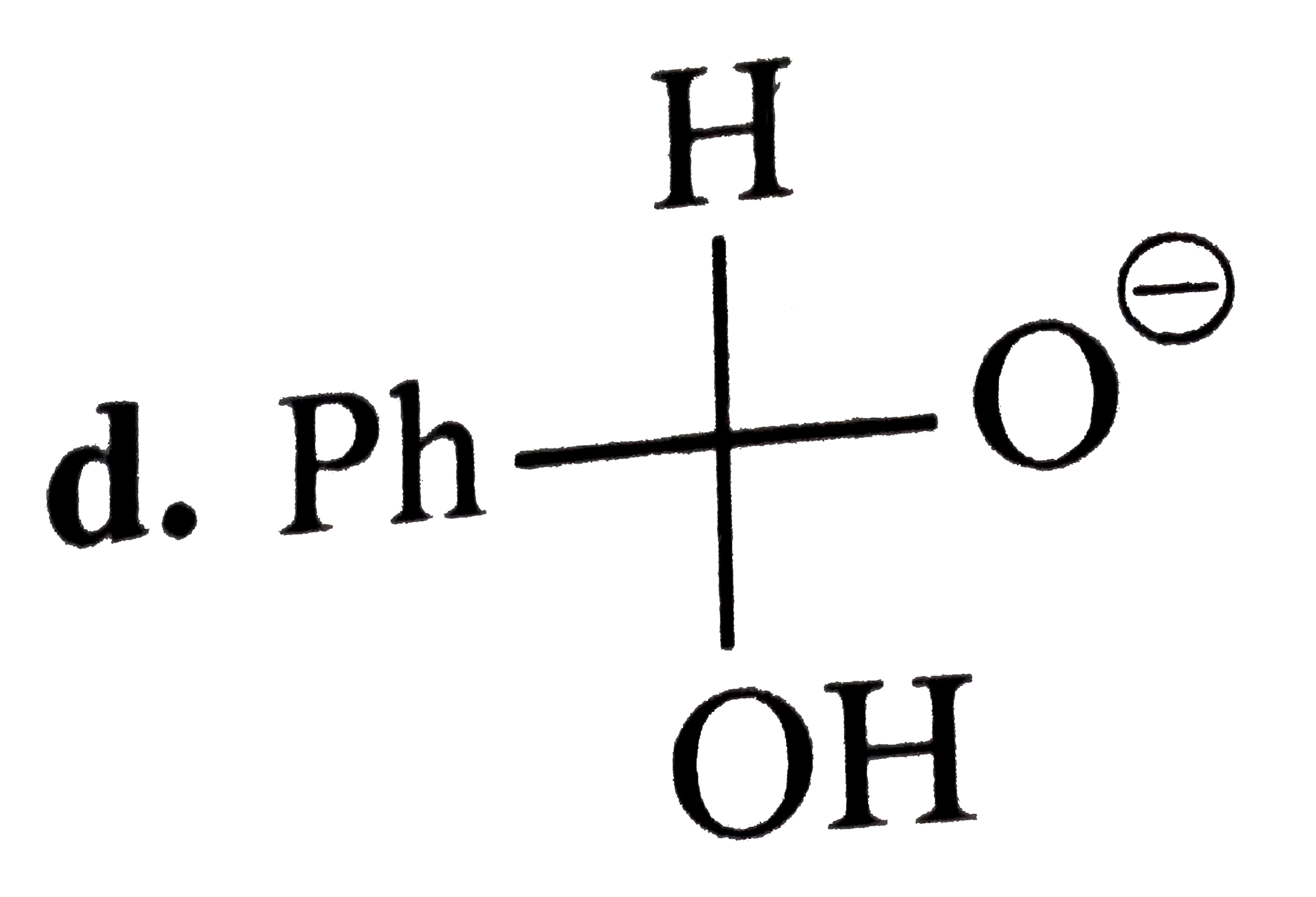
The correct Answer is:A, D
To determine the best and the least H⁻ ion donor among the given compounds, we will analyze the structures and their ability to donate hydride ions (H⁻).
1. Identify the Compounds:
Let's denote the compounds as A, B, C, and D. We need to analyze their structures to understand their ion-donating capabilities.
2. Analyze the Anions:
- Compounds B and D are dianions (having two negative charges), while A and C are anions with one negative charge each.
- Generally, dianions are more stable due to resonance stabilization, which makes them more likely to donate H⁻ ions.
3. Compare Compounds A and C:
- Compound A has two hydrogen atoms (H) available for donation, while compound C has one phenyl group and one hydrogen atom.
- The presence of two hydrogen atoms in A suggests a greater tendency to lose H⁻ compared to C, which only has one hydrogen.
4. Compare Compounds B and D:
- Both B and D are dianions. However, D has two hydrogen atoms available for donation, while B has only one.
- Therefore, D would be more likely to donate H⁻ ions compared to B.
5. Conclusion:
- The best H⁻ ion donor is compound A because it has the highest number of hydrogen atoms available for donation.
- The least H⁻ ion donor is compound D, as it has the least stabilizing factors compared to the other dianion (B).
Final Answer:
- Best H⁻ ion donor: A
- Least H⁻ ion donor: D
Let's denote the compounds as A, B, C, and D. We need to analyze their structures to understand their ion-donating capabilities.
- Compounds B and D are dianions (having two negative charges), while A and C are anions with one negative charge each.
- Generally, dianions are more stable due to resonance stabilization, which makes them more likely to donate H⁻ ions.
- Compound A has two hydrogen atoms (H) available for donation, while compound C has one phenyl group and one hydrogen atom.
- The presence of two hydrogen atoms in A suggests a greater tendency to lose H⁻ compared to C, which only has one hydrogen.
- Both B and D are dianions. However, D has two hydrogen atoms available for donation, while B has only one.
- Therefore, D would be more likely to donate H⁻ ions compared to B.
- The best H⁻ ion donor is compound A because it has the highest number of hydrogen atoms available for donation.
- The least H⁻ ion donor is compound D, as it has the least stabilizing factors compared to the other dianion (B).
- Best H⁻ ion donor: A
- Least H⁻ ion donor: D
टॉपर्स ने हल किए ये सवाल
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRY ENGLISHChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Single Correct
Explore 65 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRY ENGLISHChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Assertion-Reasoning
Explore 5 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRY ENGLISHChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Exercises Linked Comprehension
Explore 29 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Archives Analytical And Descriptive
Explore 14 Videos
- APPENDIX INORGANIC VOLUME 2Book:CENGAGE CHEMISTRY ENGLISHChapter:APPENDIX INORGANIC VOLUME 2Exercise:Short Answer Type
Explore 178 Videos
समान प्रश्न
ज्ञान जांच
Which one among the following is the least basic?
ACH3NH2B..NH3CCH3CH2NH2D
Among the following which is the least basic
View SolutionAmong the following the least soluble sulphate is
View SolutionWhich one among the following is the least basic:
View SolutionAmong the following, the least stable resonance structure is :
View SolutionWhich among the following is the least soluble in water
View SolutionAmong the following, least soluble compound in water is
View SolutionAmong the following oxides, which is least acidic ?
View Solution
