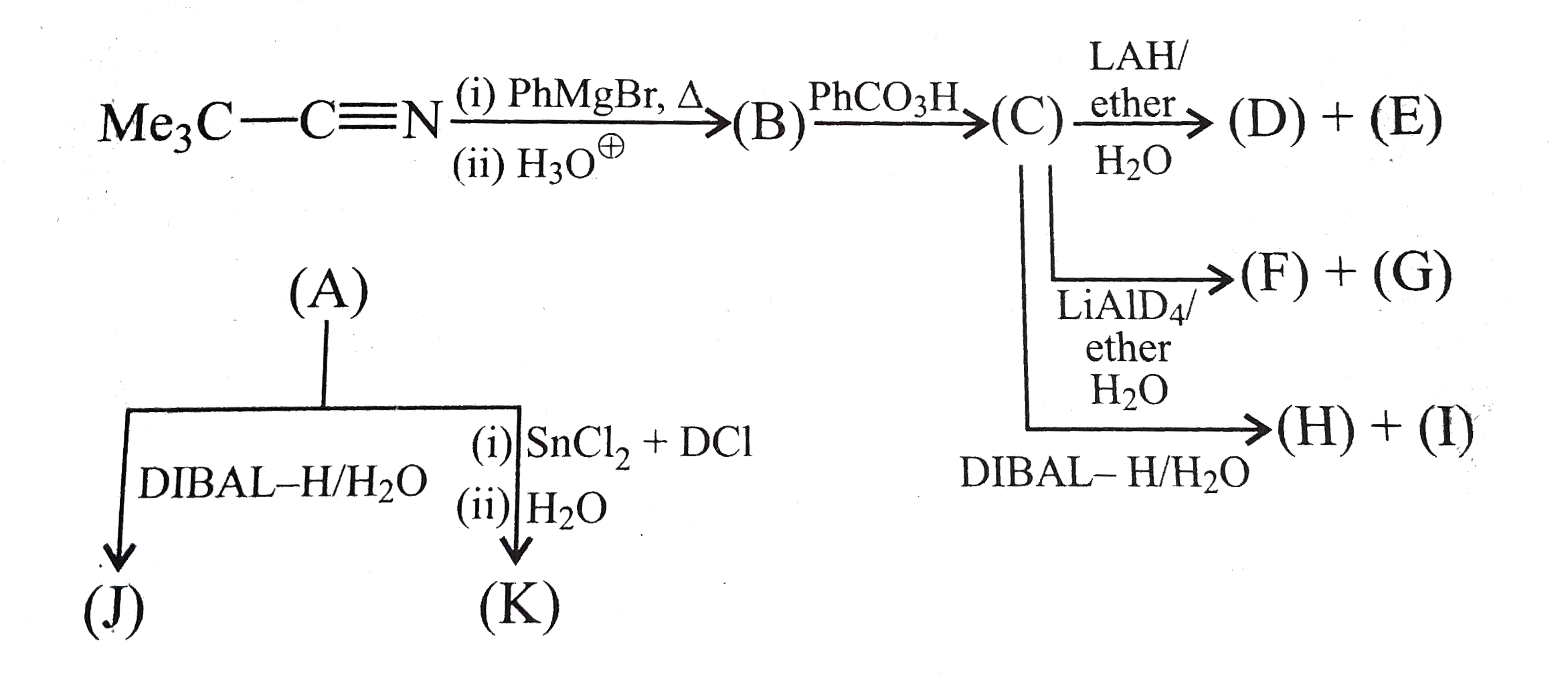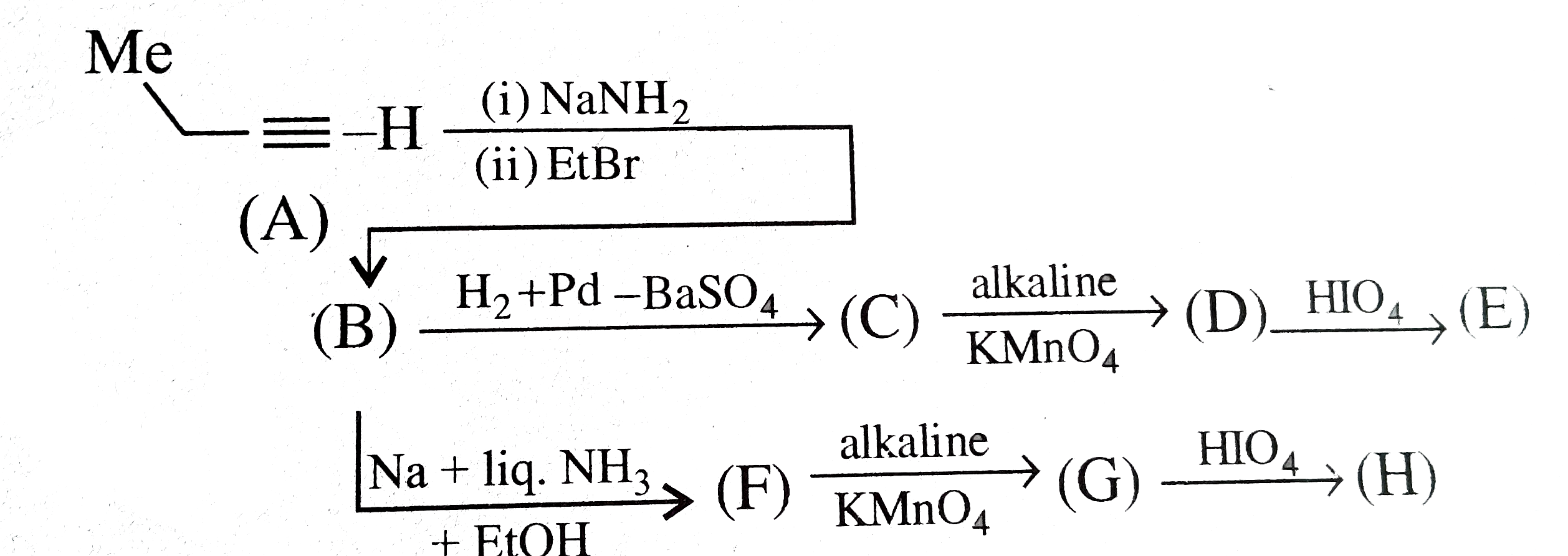
The compound (H) is:
A
a.
B
b.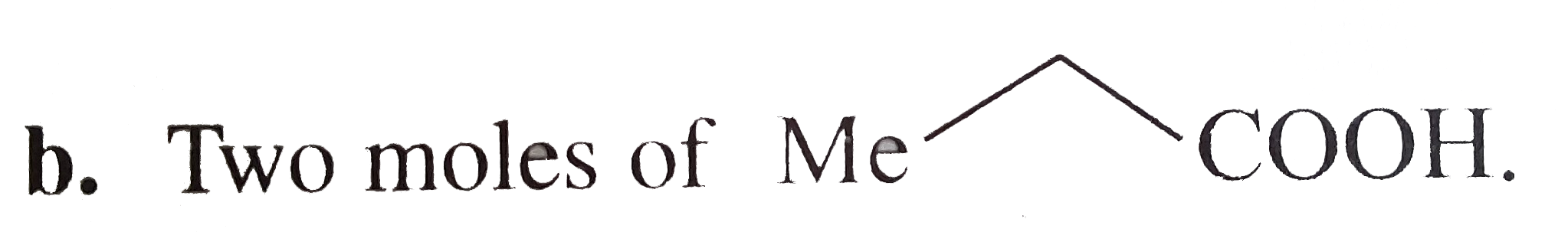
C
c.One mole of (a) and one mol of (b).
D
d. No reaction.
Video Solution
Text Solution
The correct Answer is:D
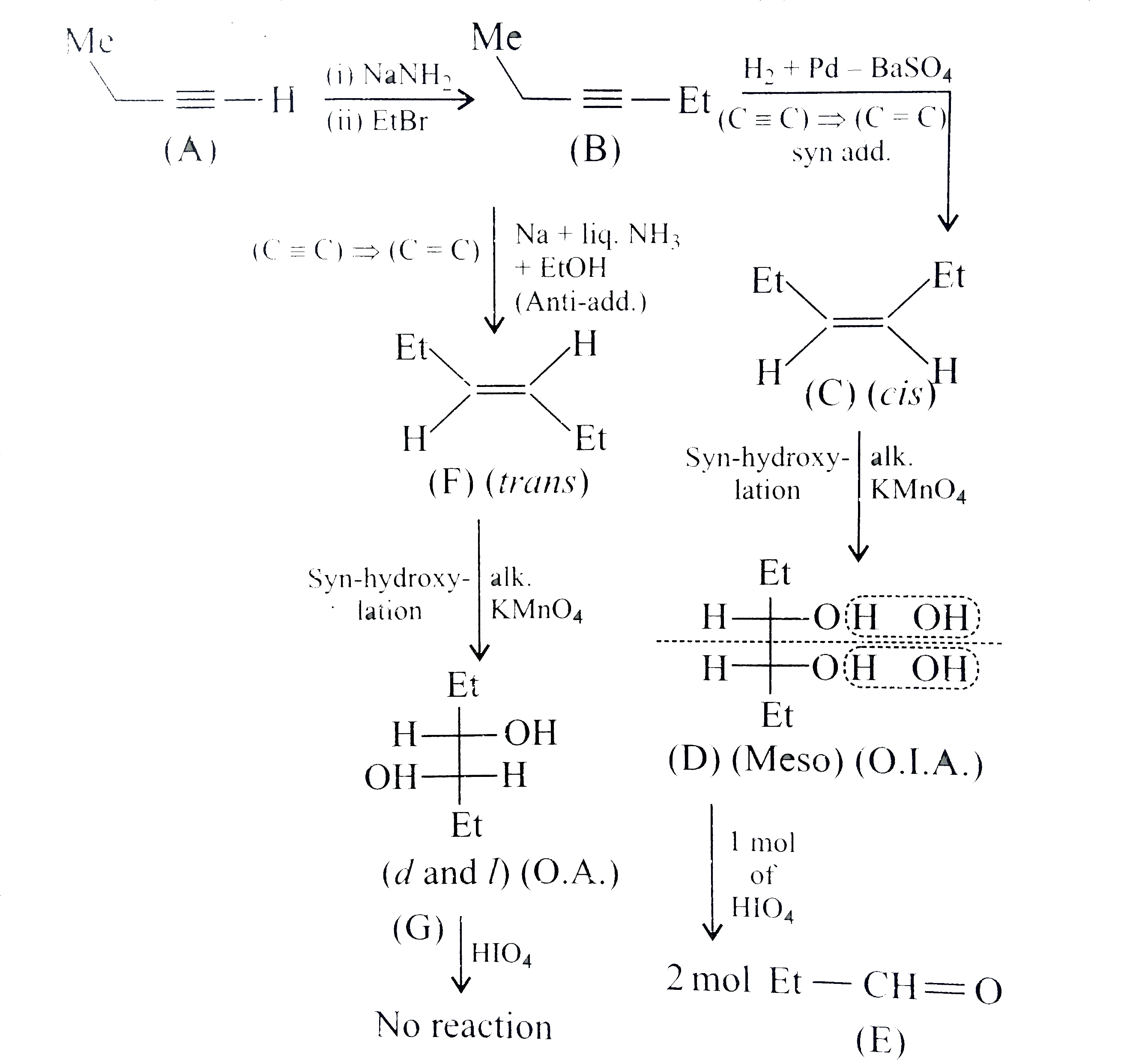
(Since two (−OH) group are in anti position, for periodic cleavage, two (−OH) must be in syn (or cis) position.)
|
Topper's Solved these Questions
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:Exercise (Multiple Correct)
Explore 23 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:Exercise (Single Correct)
Explore 84 Videos
- REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRY ENGLISHChapter:REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDSExercise:EXERCISES
Explore 1 Video
- QUALITATIVE INORGANIC SALT ANALYSISBook:CENGAGE CHEMISTRY ENGLISHChapter:QUALITATIVE INORGANIC SALT ANALYSISExercise:Viva Voce Questions And Part-C (Analysis Of Cations)
Explore 42 Videos
- SOLID STATEBook:CENGAGE CHEMISTRY ENGLISHChapter:SOLID STATEExercise:Ex 1.2 (Objective)
Explore 9 Videos
Similar Questions
Knowledge Check
Name of the compound
H3C−CH3∣C∣CH3−CH=CH2
as per IUPAC system isA3, 3, 3-trimethyl-1-propaneB1, 1, 1-trimethyl-3-propanoneC3, 3-dimethylbut-1-eneD1, 1-dimethyl-3-butene.
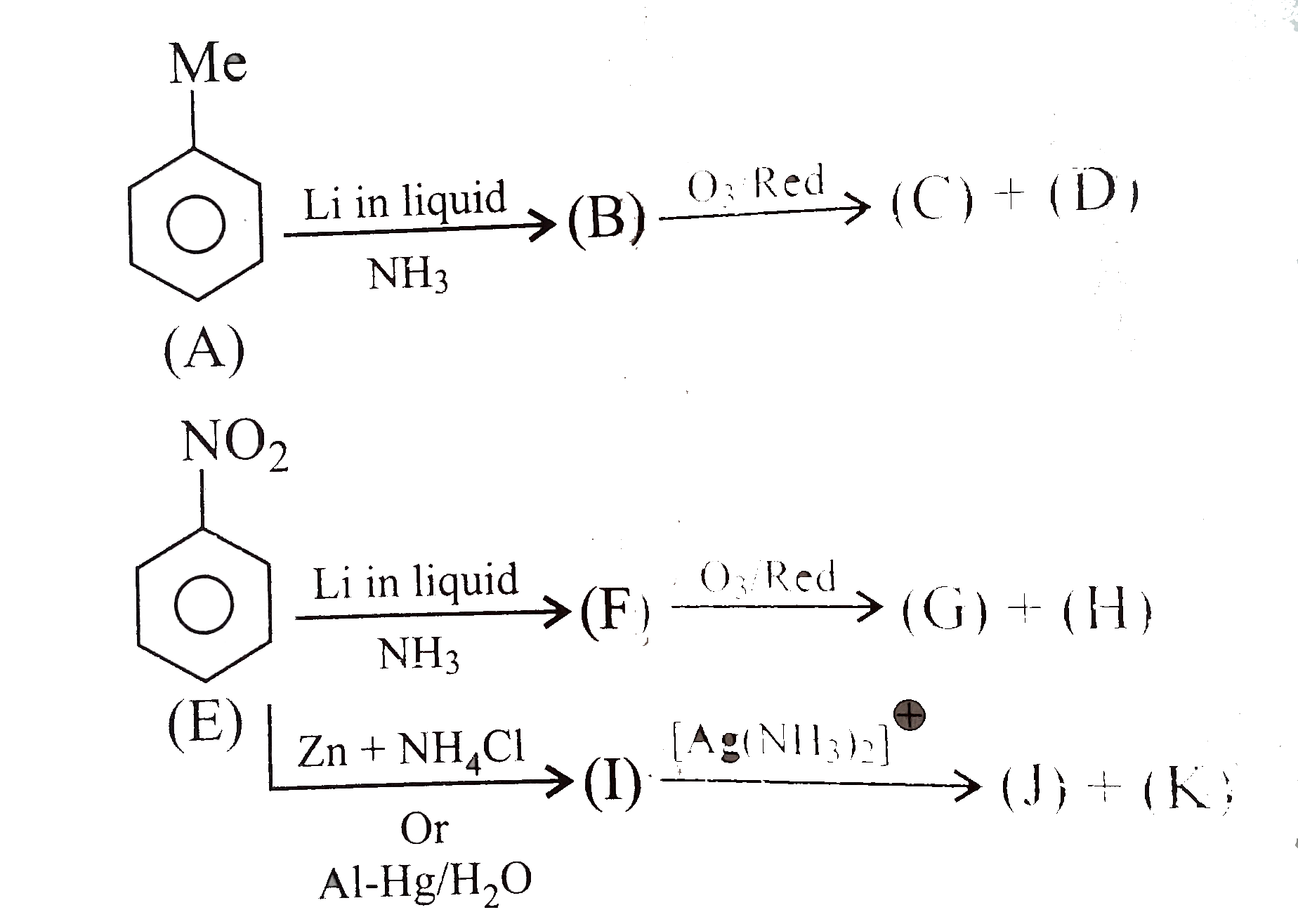
The compound (G) and (H), respectively, are:
View Solution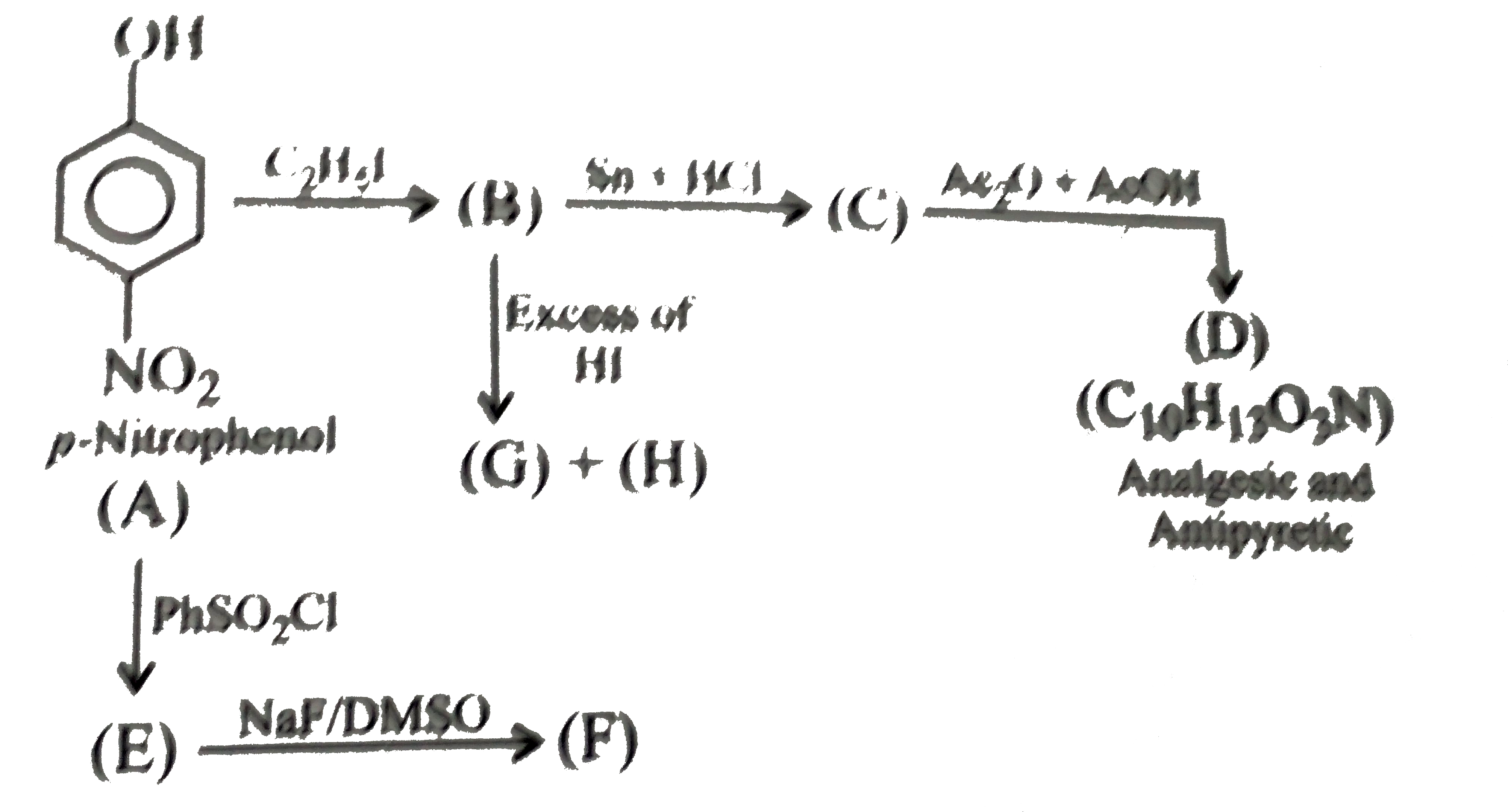
The compound (G) and (H), respectively, are:
View SolutionThe compound H2N−Hg−O−Hg−I is formed by the action of
View Solution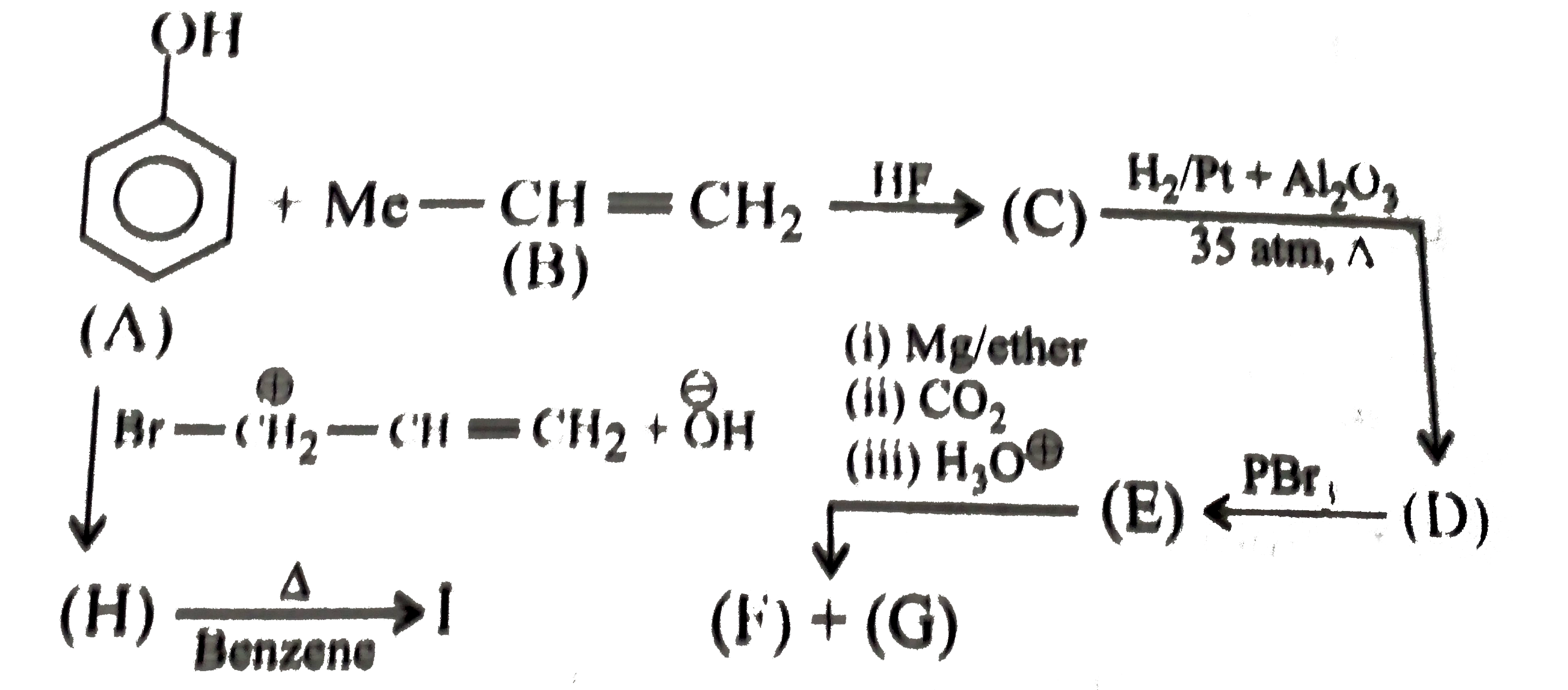
The reaction and mechanism involved in the formation of compound (H) form (A), respectively, are:
View SolutionThe correct IUPAC name of the compound
H3C−C=CH−CH−CH3 | | Cl CH3
View SolutionTotal number of stereoisomer possible for the compound.
H3C−CH=CH−CH=CH−CH=CH−Ph
View SolutionThe E.N. of H,X,O are 2.1,0.8 and 3.5 respectively comment on the nature of the compound H−O−X, that is :
View Solution