Write the structure of the major organic product in each of the following reactions:
a. CH3CH2CH2Cl+NalAccetoneHeat
b. (CH3)3CBr+KOHEthanolHeat
c. CH3CH(Br)CH2CH3+NaOHWater−−−→
<br>d.CH_(3)CH_(2)CH = CH_(2) + HBr overset("Peroxide")
a. 

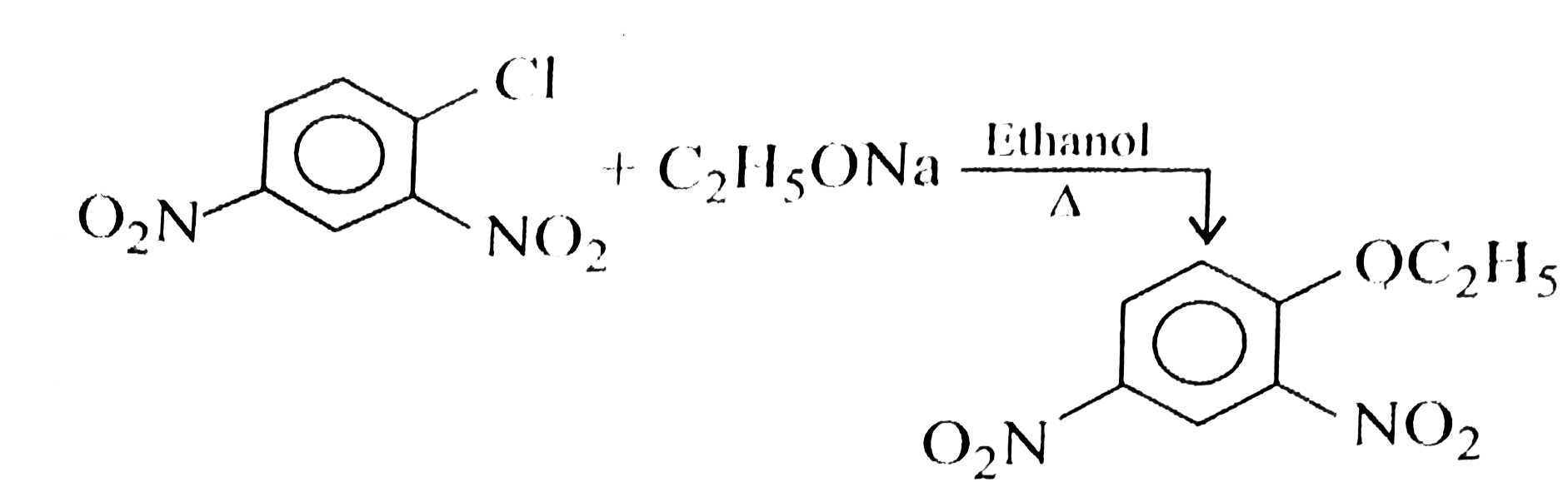
(Due to two electric withdrawing groups [(NO2) group], SN reactivity increases and hence reaction takes place with aryl halide.)
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Concept Application
4 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Linked Compreshension
40 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES EXERCISE - Exercises Subjective Type
1 Video
- APPENDIX INORGANIC VOLUME 2BOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - APPENDIX INORGANIC VOLUME 2EXERCISE - Short Answer Type
178 Videos
- BIOMOLECULESBOOK - CENGAGE CHEMISTRY ENGLISHCHAPTER - BIOMOLECULESEXERCISE - Exercises Archives (Analytical And Descriptive)
5 Videos
Similar Questions
How will you bring about the following conversations in not more than two steps?
a. Propene to 1− Nitropropane
b. Bromocethane to cis-hex −3− ene
View SolutionParagraph for problems 9−15
Compound (A) on reaction with NH2NH2+o−OH is:
View SolutionA white precipitate was formed slowly when silver nitrate was added to compound (A) with molecular formula C6H13Cl.Compound (A) on treatment with hot alcoholic potassium hydroxide gave a misture of two isomeric alkenes (B) and (C), havinig the formuls C6H12. The mixture of (B) and (C), on ozonolsis, furnished four compounds.
i. CH3CHO,ii C2H5CHO
iii. CH3COCH3,(iv) CH3−CH∣CH3−CHO
What are the structures of (A),(B)and (C) ?
View SolutionWhich metals are generally extracted by the electrolytic processes ? What positions these metals generally occupy in the periodic table?
View SolutionAlanine, the amino acid the respective structure at pH = 2 and pH = 10 will be
View SolutionGiven the IUPAC names of the following compounds: (i) CH_(3)CH_(2)CH_(2)underset(C_(2)H_(5))underset(|)overset(CH_(3))underset(|)(C )CH_(2)-overset(CH_(3))overset(|)(C )=CH_(2) (ii) CH_(3)-overset(CH_(3))overset(C )H-C-=CH (iii) C_(6)H_(5)-CH=CH-CH_(2)Cl CH_(3)CH-CH-CH_(2)Cl (iv) CH_(3)CH=CH-CH_(2)Br v CH_(2)=CH-overset(Cl)overset(|)(C )-CH=CH_(2) vi CH_(2)=CH-overset(CH_(2)CH_(3))underset(|)(C )H-underset(Cl)underset(|)(C )=CH_(2) vii. (CH_(3))_(3)C - CH=CH_(2)
View SolutionWrite IUPAC name of the following compound (a) CH_(3)-CH_(2)-underset(CH_(3))underset(|)(CH)-CH_(2)underset(CH_(2)-CH_(3))underset(|)(CH)-CH_(2)-CH_(2)-CH_(3) (b) CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-CH_(2)-overset(CH_(2)-CH_(3))overset(|)(CH)-CH_(2)-underset(CH_(3))underset(|)(CH)-CH_(3) (c)
View SolutionWrite the IUPAc name of the following compound :
(a) CH3−CH2−CH3∣CH−CH=CH−CH2−CH3
(b) CH3−CH2−CH3∣CH−CH2−CH3∣CH−CH∣CH2∣CH2∣CH−CH3∣CH3−CH=CH3∣C−CH3
View SolutionCH3−C∣CH3=CH2HBr−−−−→PeroxideCH3−CH∣CH3−CH2Br reaction intermediate of this reaction is:
View SolutionThe IUPAC name of the compound CH_(3)CH_(2)CH_(2)underset(CH(CH_(3))_(2))underset(|)(CH)-overset(CH_(3))overset(|)(CH)-underset(CH_(2)CH_(3))underset(|)overset(CH_(2)CH_(3))overset(|)(C)-CH_(2)CH_(3)
View Solutionनिम्नलिखित यौगिकों के IUPAC नाम लिखिए-
(a) CH3−Br∣CH−Br∣CH−COOC2H5
(b) (CH3)3C.CH2.CH2Cl
(c) CH3−CH2−CH∣Br−CH∣CH3−CH3∣C∣CH3−CH3
(d) CH3−CH2−CH∣Br−CH3∣C∣CH3−CH2Cl
View Solutionनिम्नलिखित अभिक्रियाओं के प्रमुख उत्पाद कि संरचना लिखिए -
(i) CH3CH2CH2Cl+NaIAcetone−−−−−−→Heat
(ii) (CH3)3CBr+KOHEthanol−−−−−−→Heat
(iii) CH3CH(Br)CH2CH3+NaOHWater−−−→
(iv) CH3CH2Br+KCNAq. ethanol−−−−−−→
(v)C6H5ONa+C2H5Cl→
(vi) CH3CH2CH2OH+SOCl2→
(vii) CH3CH2CH=CH2+HBrPeroxide−−−−→
(viii) CH3CH=C(CH3)2+HBr→
View SolutionWrite the structure of the major product and IUPAC name in each of the following reactions :
(i) CH3CH2CH2Cl+NaIAcetone−−−−→Heat (ii) CH3CH2Cl+SbF3Heat−−→
(iii) CH3CH2CH2OH+SOCI2→ (iv) CH3CH2CH=CH2+HBrPeroxide−−−−→
(v) CH3CH2C∣CH3=CH2+HBrNo Peroxide−−−−−−→ (vi) CH3CH2CH2Br+Hg2F2→
(vii) CH3CH2C≡CH+HCl(1-equiv)→ (viii) CH3CH=CH2+Cl2773−873k−−−−−→
View SolutionWrite the structure of the major product and IUPAC name in each of the following reactions :
(i) CH3CH2CH2Cl+NaIAcetone−−−−→Heat
(ii) CH3CH2Cl+SbF3Heat−−→
(iii) CH3CH2CH2OH+SOCl2→
(iv) CH3CH2CH=CH2+HBrPeroxide−−−−→
(v) CH3CH2C∣CH3=CH2+HBrNo Peroxide−−−−−−→
(vi) CH3CH2CH2Br+Hg2F2→
(vii) CH3CH2C≡CH+HCl(1-equiv)→
(viii) CH3CH=CH2+Cl2773−873k−−−−−→
View SolutionName the following compounds accoring to IUPAC system : (i) CH_(3)-CH_(2)- underset(CH_(2)Cl) underset(|)(CH)-overset(CH_(2)OH) overset(|)(CH)-underset(CH_(3)) underset(|)(CH)-CH_(3) (ii) CH_(3)-underset(CH_(3))underset(|)(CH)-CH_(2)-underset(OH) underset(|)(CH)-overset(CH_(2)OH) overset(|)(CH)-CH_(3) (iii) (iv) H_(2)C=CH-underset(OH)underset(|)(CH)-CH_(2)-CH_(2)-CH_(3) (v) CH_(3)- underset(CH_(3)) underset(|)(C)= underset(Br) underset(|)(C)-CH_(2)OH
View Solution

