Consider the folliwng reactions:
I. Me3C−BrH2O+NaBr−−−−−−−−−→SN1Product
II. Ph3C−BrH2O+NaBr−−−−−−−−−→SN1Products
Which of the folliwng statements are correct about the above reactions?
The products in reactions (I) and (II) are mixture of (Me3−OH+Me3Br) and (Ph3C−OH+Ph3C−Br), respectively.
The product is (I) is (Me3C−OH) and in (II) is (Ph3C−OH+Ph3C−Br).
The product in (I) is (Me3C−OH+Me3C−Br0 and in (II) is (Ph3C−OH).
Ph3C⊕ is more stable than MeC⊕.
The correct Answer is:(b,d)
To analyze the given reactions and determine the correct statements, we will break down the reactions step by step.
Step 1: Analyze Reaction I
The first reaction involves a tertiary alkyl bromide, Me3C−Br, undergoing an SN1 reaction with water (H2O) and sodium bromide (NaBr).
1. Formation of Carbocation: The tertiary alkyl bromide will first undergo ionization to form a tertiary carbocation (Me3C+). This step is the rate-determining step in an SN1 mechanism.
Hint: Tertiary carbocations are more stable due to hyperconjugation and inductive effects.
2. Nucleophilic Attack: The water molecule acts as a nucleophile and attacks the carbocation, leading to the formation of a tertiary alcohol (Me3C−OH).
Hint: In SN1 reactions, the nucleophile attacks the carbocation after its formation.
Step 2: Analyze Reaction II
The second reaction involves a triphenylmethyl bromide, Ph3C−Br, also undergoing an SN1 reaction with water and sodium bromide.
1. Formation of Carbocation: The triphenylmethyl bromide will ionize to form a benzylic carbocation (Ph3C+). This carbocation is highly stabilized by resonance due to the three phenyl groups attached to it.
Hint: Resonance stabilization significantly increases the stability of carbocations.
2. Nucleophilic Attack: Similar to the first reaction, water will attack the benzylic carbocation, forming triphenylmethanol (Ph3C−OH). However, due to the partial double bond character of the carbon-bromine bond in aryl halides, the reaction is less favorable, and the product may be formed in minor amounts.
Hint: Aryl halides are less reactive in SN1 reactions due to the strength of the C-Br bond.
Step 3: Evaluate the Statements
Now we will evaluate the statements based on our analysis of the reactions:
1. Statement A: Product one is a mixture of products.
- Evaluation: This statement is incorrect. Product one is a single tertiary alcohol (Me3C−OH).
2. Statement B: Product one is a tertiary alcohol while product two is a mixture.
- Evaluation: This statement is correct. Product one is indeed a tertiary alcohol, and product two (triphenylmethanol) is formed in minor amounts.
3. Statement C: Benzyl carbocation is more stable than tertiary carbocation.
- Evaluation: This statement is correct. The benzylic carbocation is resonance-stabilized by three phenyl groups, making it more stable than the tertiary carbocation.
4. Statement D: The reaction of triphenylmethyl bromide does not proceed via SN1 due to the partial double bond character.
- Evaluation: This statement is partially correct. While the reaction does proceed via SN1, it is less favorable due to the strong C-Br bond in aryl halides.
Final Conclusion
Based on the evaluations:
- Correct Statements: B and C.
- Incorrect Statements: A and D.
Summary of the Solution
1. Reaction I: Forms a tertiary alcohol (Me3C−OH).
2. Reaction II: Forms triphenylmethanol (Ph3C−OH) but is less favorable due to the strength of the C-Br bond.
3. Correct Statements: B and C.
The first reaction involves a tertiary alkyl bromide, Me3C−Br, undergoing an SN1 reaction with water (H2O) and sodium bromide (NaBr).
Hint: Tertiary carbocations are more stable due to hyperconjugation and inductive effects.
The second reaction involves a triphenylmethyl bromide, Ph3C−Br, also undergoing an SN1 reaction with water and sodium bromide.
Now we will evaluate the statements based on our analysis of the reactions:
- Evaluation: This statement is incorrect. Product one is a single tertiary alcohol (Me3C−OH).
- Evaluation: This statement is correct. Product one is indeed a tertiary alcohol, and product two (triphenylmethanol) is formed in minor amounts.
- Evaluation: This statement is correct. The benzylic carbocation is resonance-stabilized by three phenyl groups, making it more stable than the tertiary carbocation.
- Evaluation: This statement is partially correct. While the reaction does proceed via SN1, it is less favorable due to the strong C-Br bond in aryl halides.
Based on the evaluations:
- Correct Statements: B and C.
- Incorrect Statements: A and D.
1. Reaction I: Forms a tertiary alcohol (Me3C−OH).
2. Reaction II: Forms triphenylmethanol (Ph3C−OH) but is less favorable due to the strength of the C-Br bond.
3. Correct Statements: B and C.
Topper's Solved these Questions
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRY ENGLISHChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Multiple Correct Answers Type
Explore 8 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRY ENGLISHChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Single Correct
Explore 139 Videos
- AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Book:CENGAGE CHEMISTRY ENGLISHChapter:AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES Exercise:Exercises Linked Compreshension Type
Explore 8 Videos
- APPENDIX INORGANIC VOLUME 2Book:CENGAGE CHEMISTRY ENGLISHChapter:APPENDIX INORGANIC VOLUME 2Exercise:Short Answer Type
Explore 178 Videos
- BIOMOLECULESBook:CENGAGE CHEMISTRY ENGLISHChapter:BIOMOLECULESExercise:Exercises Archives (Analytical And Descriptive)
Explore 5 Videos
Similar Questions
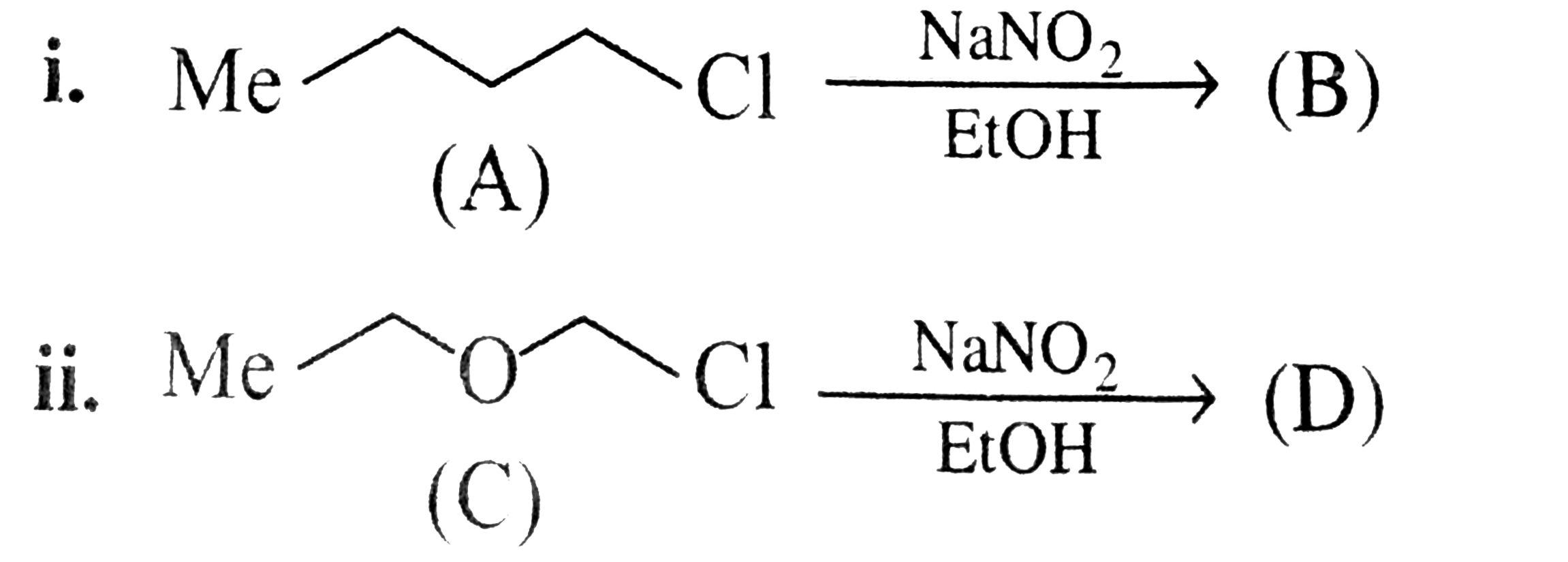
Which of the following statements is/are correct for the above reactions?
View SolutionThe decreaing order of the rate of niration of the following compounds is
I. Benzene, II. C6D6
III. Nitrobenzene, IV Chlorobenzene
View SolutionA hydrocation (A)(C8H10)(E) is a stream volatile compound and on nitration gives two mononitro derivatives. (A) gives the following reactions.
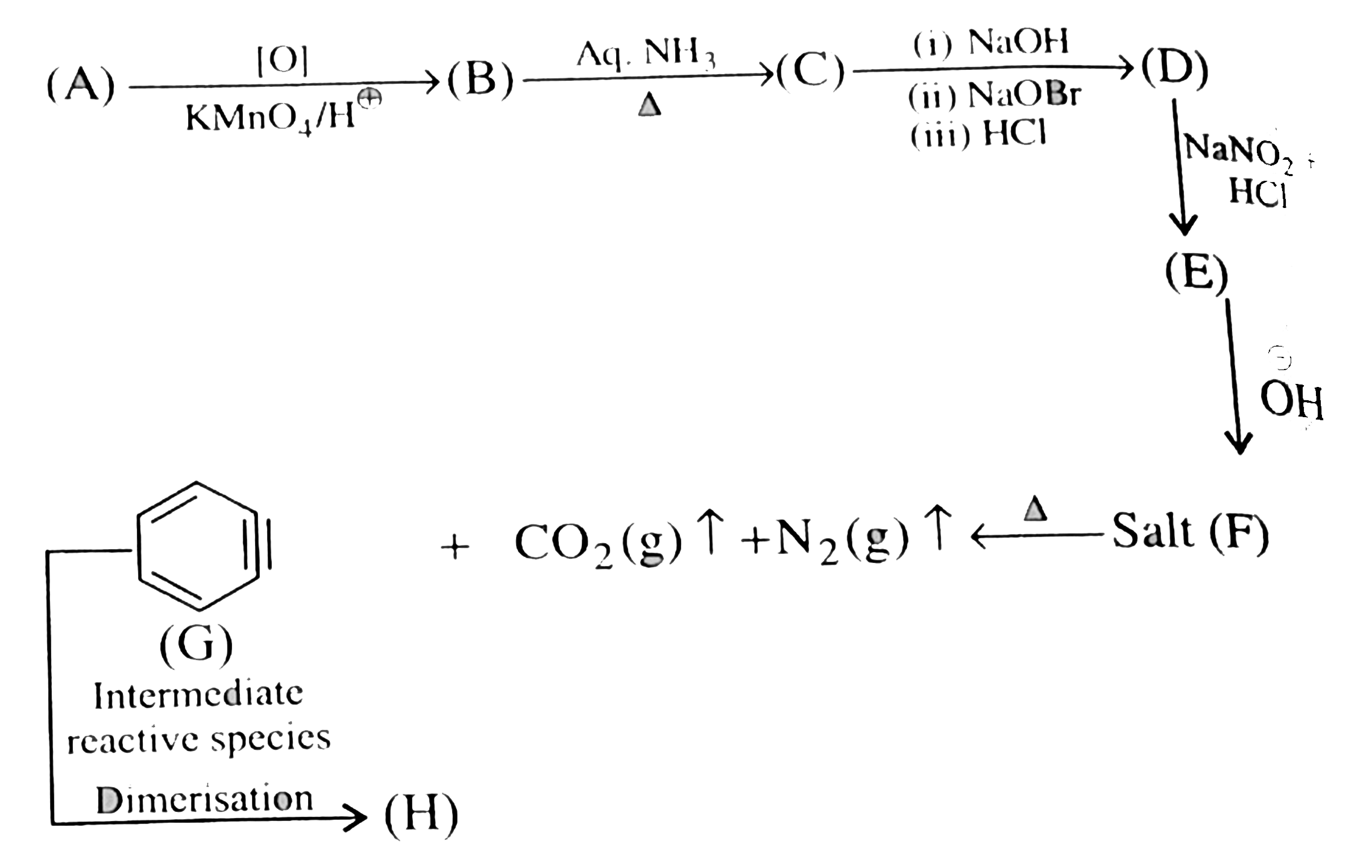
Compound (A) is:
View SolutionWhich metals are generally extracted by the electrolytic processes ? What positions these metals generally occupy in the periodic table?
View SolutionAlanine, the amino acid the respective structure at pH = 2 and pH = 10 will be
View Solutionoverset(1.Br_(2)^(-)+KOH, Delta)underset(2.H_(3)O^(o+))rarr The product of above reaction is:
View SolutionCH_(3)-underset(Br)underset(|)CH-CH_(2)-overset(O)overset(||)C-CH_(3)underset(Delta)overset(N_(2)H_(4),overset(Ө)OH)rarr product :
View SolutionComplete the following reaction sequence : CH_(3)-overset(O)overset(||)C-CH_(3)overset((i)CH_(3)MgBr)underset((ii)H_(2)O)rarr(A)overset("Na metal")underset("ether")rarr(B)overset(CH_(3)-Br)rarr(C)
View SolutionConsider the following reaction. CH_(3)overset(O^(16))overset(||)C-O^(18)-C_(2)H_(5)+H_(2)O^(16)underset("Heat")overset(H^(+))rarr The products formed in the reaction are
View SolutionIn the reaction sequence CH_(3)-overset(O)overset(||)(C)-H-underset(.^(Θ)OH)overset(HCN)rarr(A) underset(Delta)overset(H_(2)O //overset(o+)(H))rarr Product is/are :
View SolutionConsider the folliwng reactions: I. Me_(3)C - Br underset(SN^(1))overset(H_(2)O + NaBr)(rarr) "Product" II. Ph_(3)C - Br underset(SN^(1))overset(H_(2)O + NaBr)(rarr) "Products" Which of the folliwng statements are correct about the above reactions?
View Solution"But-1-ene"overset((CH_(3)COO)_(2)Hg)underset(H_(3)O)rarr? The product in the above reaction is
View SolutionIn the following sequence of reaction, the product C is CH_(3)-Br overset(KCN) rarr A overset(H_(2)O^(+))rarr B overset(LiAlH_(4))underset("Ether")rarr C
View SolutionIn the reaction : CH3−Br∣C∣Br−Br∣C∣Br−HZn−−→ the product is :
View SolutionCH3C≡CCH3(i)X−−−−−−−−−→(ii)H2O/ZnH3C−C∣∣O−C∣∣O−CH3
In the above reaction X is
View Solution
