A (He)+ ion in ground state is fired towards a Hydrogen atom in'the ground state and at rest. What should be the minimum kinetic energy (in eV ) of (He)+ ion so that both the single electron species may get excited?
The correct Answer is:255
Topper's Solved these Questions
Similar Questions
The continuous x - ray spectrum obtained from a Coolidge tube is of the form
View SolutionEach capacitance shown in is in μF. Find the charge on 6μC.
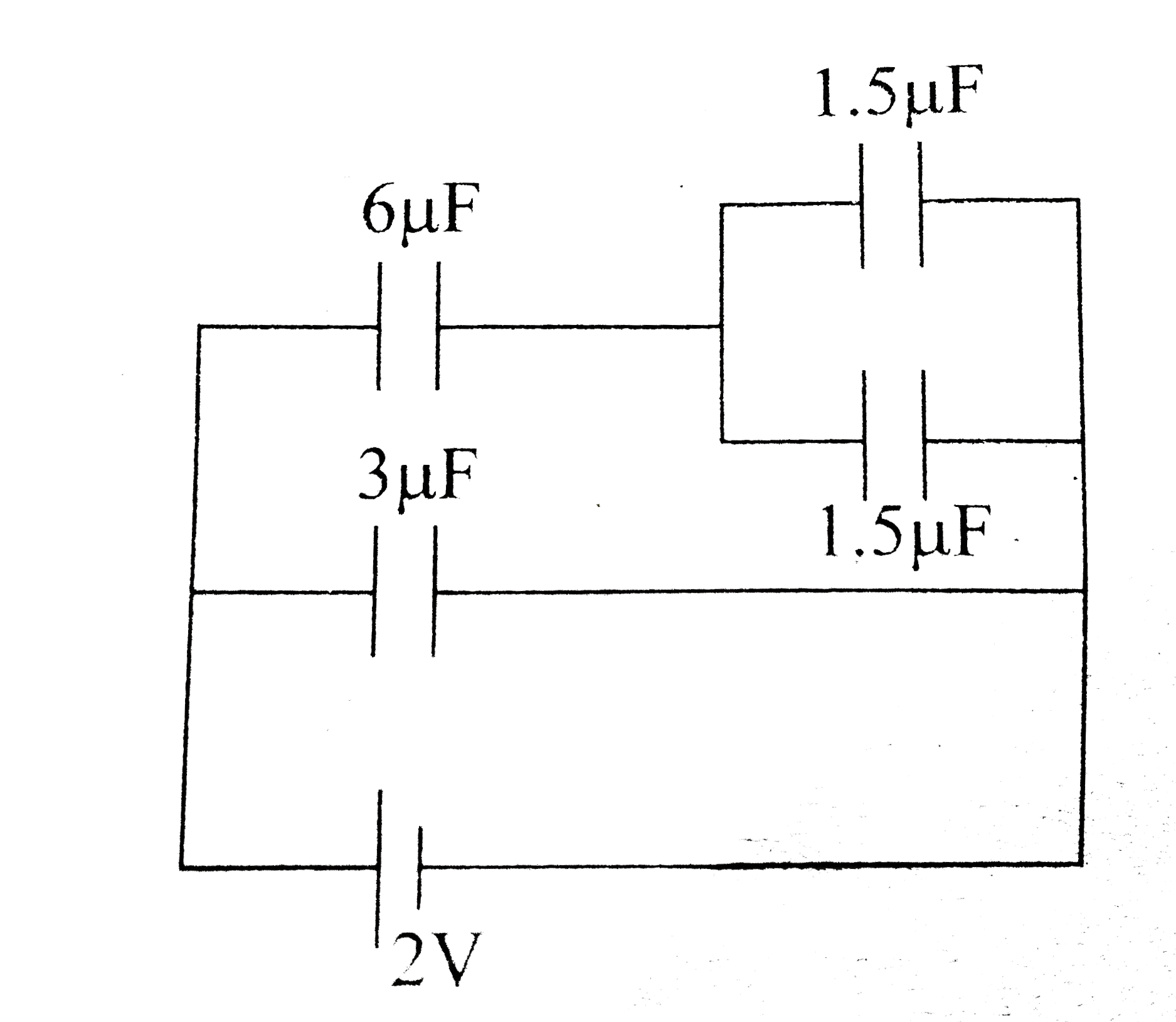 .
.
View SolutionThe excited energy of a He+ ion is the same as the ground state energy of hydrogen is it always true that one of the energies of any hydrogen like ion will bethe same as the ground state energy of a hydrogen atom?
View SolutionThe energy of a hydrogen atom in the ground state is -13.6 eV . The eneergy of a He^(+) ion in the first excited state will be
View SolutionThe ground state energy of hydrogen atom is −13.6eV. The energy of second excited state of He+ ion in eV is
View SolutionShow that ground state energy of electron in hydrogen atom is equal to the first excited state energy of electron in He^(+) ion, assuming that their Rydberg's constants are equal
View SolutionWhat is the ratio of energy of the ground state of hydrogen atom and energy of the first excited state of He^+ ion (Z=2) ?
View SolutionThe energy of a hydrogen atom in the ground state is -13.6 eV. The energy of a He+ ion in the first excited state will be
View SolutionIf potential energy of an electron in a hydrogen atom in first excited state is taken to be zero kinetic energy (in eV) of an electron in ground state will be
View SolutionIn a hydrogen atom , If the energy of electron in the ground state is -x eV ., then that in the 2nd excited state of He+ is
View SolutionThe total energy of electron in the ground state of hydrogen atom is (-13.6 eV) . The kinetic energy of an electron in the first excited state is
View SolutionExcitation energy of a hydrogen like ion in its first excitation state is 40.8 eV. Energy needed to remove the electron from the ion in ground state is
View Solutionहाइड्रोजन परमाणु की मूल अवस्था ऊर्जा - 13.6 eV है। He+ आयन की द्वितीय उत्तेजित अवस्था की ऊर्जा, eV में, है
View SolutionThe energy of a hydrogen atom in the ground state is -13.6 eV. The energy of a He+ ion in the first excited state will be .....
View SolutionThe total energy of electron in the ground state of hydrogen atom is 13.6 eV. Find the kinetic energy of an electron in the first excited state
View Solution

