According to Mendeleev's periodic law physical and chemical properties are function of
Option 1. Atomic number
Option 2. Atomic weight
Option 3. Atomic volume
Option 4. Number of neutrons
The correct Answer is:B
To answer the question regarding Mendeleev's periodic law, we can follow these steps:
1. Understanding Mendeleev's Periodic Law:
- Mendeleev's periodic law states that the physical and chemical properties of elements are periodic functions of their atomic weights. This means that when elements are arranged in order of increasing atomic weight, elements with similar properties appear at regular intervals.
2. Arrangement of Elements:
- Mendeleev arranged the elements in a table format where elements were placed in rows (periods) and columns (groups) based on their atomic weights. The elements in the same column share similar chemical and physical properties.
3. Identifying the Key Concept:
- The key concept here is that the properties of elements are not random but follow a periodic trend based on their atomic weights. This periodicity allows for the prediction of properties of undiscovered elements.
4. Conclusion:
- Therefore, according to Mendeleev's periodic law, the physical and chemical properties of elements are a function of their atomic weights.
Final Answer:
According to Mendeleev's periodic law, physical and chemical properties are a function of atomic weight.
---
- Mendeleev's periodic law states that the physical and chemical properties of elements are periodic functions of their atomic weights. This means that when elements are arranged in order of increasing atomic weight, elements with similar properties appear at regular intervals.
- Mendeleev arranged the elements in a table format where elements were placed in rows (periods) and columns (groups) based on their atomic weights. The elements in the same column share similar chemical and physical properties.
- The key concept here is that the properties of elements are not random but follow a periodic trend based on their atomic weights. This periodicity allows for the prediction of properties of undiscovered elements.
- Therefore, according to Mendeleev's periodic law, the physical and chemical properties of elements are a function of their atomic weights.
According to Mendeleev's periodic law, physical and chemical properties are a function of atomic weight.
Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:AAKASH INSTITUTE ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:Assignment (Section -C)
Explore 12 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:AAKASH INSTITUTE ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:Assignment (Section -D Linked Compreshension Type Questions)
Explore 6 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESBook:AAKASH INSTITUTE ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIESExercise:Assignment (Section -A)
Explore 40 Videos
- CHEMISTRY IN EVERYDAY LIFE Book:AAKASH INSTITUTE ENGLISHChapter:CHEMISTRY IN EVERYDAY LIFE Exercise:Assignment ( SECTION - A)
Explore 45 Videos
- COORDINATION COMPOUNDS Book:AAKASH INSTITUTE ENGLISHChapter:COORDINATION COMPOUNDS Exercise:Assignment (Section-J Aakash Challenger Questions )
Explore 10 Videos
Similar Questions
Choose the correct pair regarding ionisation energy (IE1)
View SolutionIonisation energy is the amount of energy required to remove the outermost e− from a gaseous atom. It's unit is kJ/mole or kcal/mole.
Successive ionisation energy - It is the amount of energy required to remove electron successively from a gaseous ion. These are termed as IE2,IE3,IE4 etc. the difference in the values of IE1,IE2 and IE3 helps to determine electronic configuration of the element.
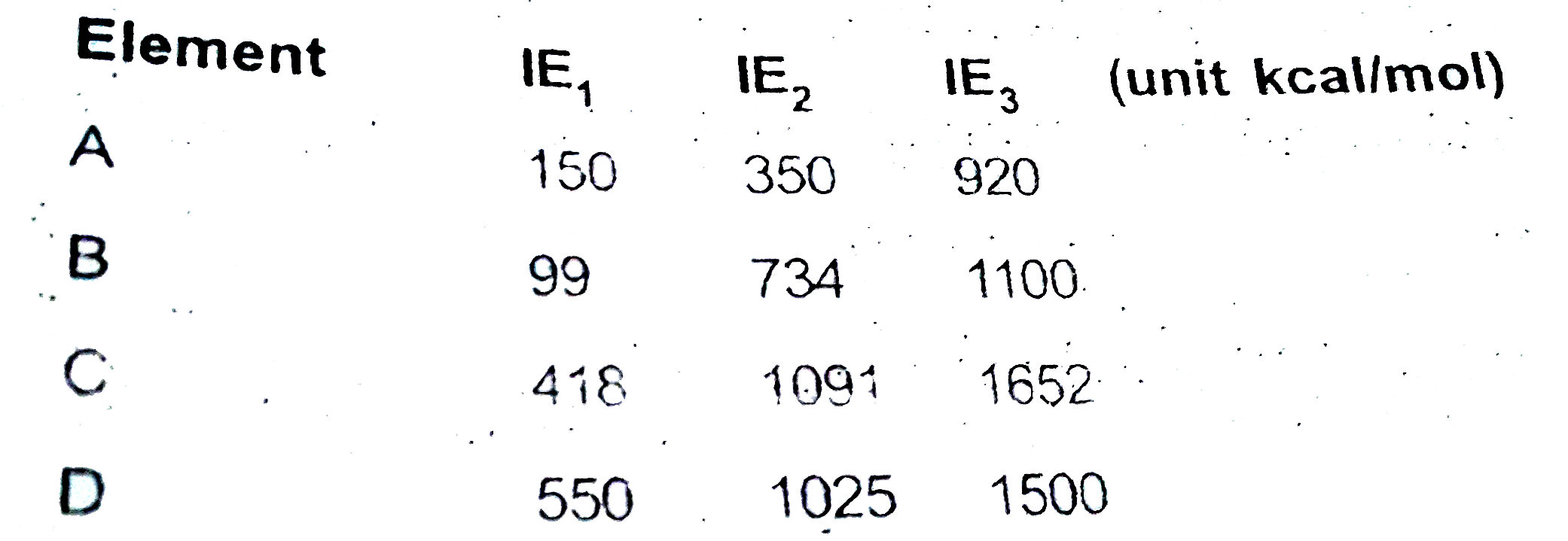
Which element is a noble gas ?
View SolutionOn moving from left to right across a period in the periodic table the metallic character
View SolutionChemotherapy is
View SolutionFor Ag+ metal ion, correct sequence regarding ligand strength is
View SolutionAs per the modern periodic law, the physical and chemical properties of elements are periodic functions of their
View SolutionAccording to Mendeleev's periodic law physical and chemical properties are function of
View SolutionAccording to Mendeleev's perodic law, the properties of elements are a periodic function of their
View SolutionAccording to Mendeleev's periodic table , the physical and chemical properties of elements are periodic function of their __________
View SolutionAs per the Modern periodic law, the physical and chemical properties of elements are periodic functions of their
View SolutionAccording to modern periodic law, the physical and chemical properties of elements are the periodic functions of their ?
View SolutionAccording to Mendeleev's periodic law, the properties of elements are a periodic function of their atomic numbers.
View SolutionAccording to Mendeleev's periodic table , the physical and chemical properties of elements are periodic function of their __________
View SolutionAccording to modern periodic law, the physical and chemical properties of the elements are periodic function of their:
View SolutionModern periodic law states that the physical and chemical properties of elements are the periodic functions of their……………….
View SolutionMoseley's periodic law: The physical and chemical properties of elements are the periodic function of their
View Solution
