Match Column-I with Column-II
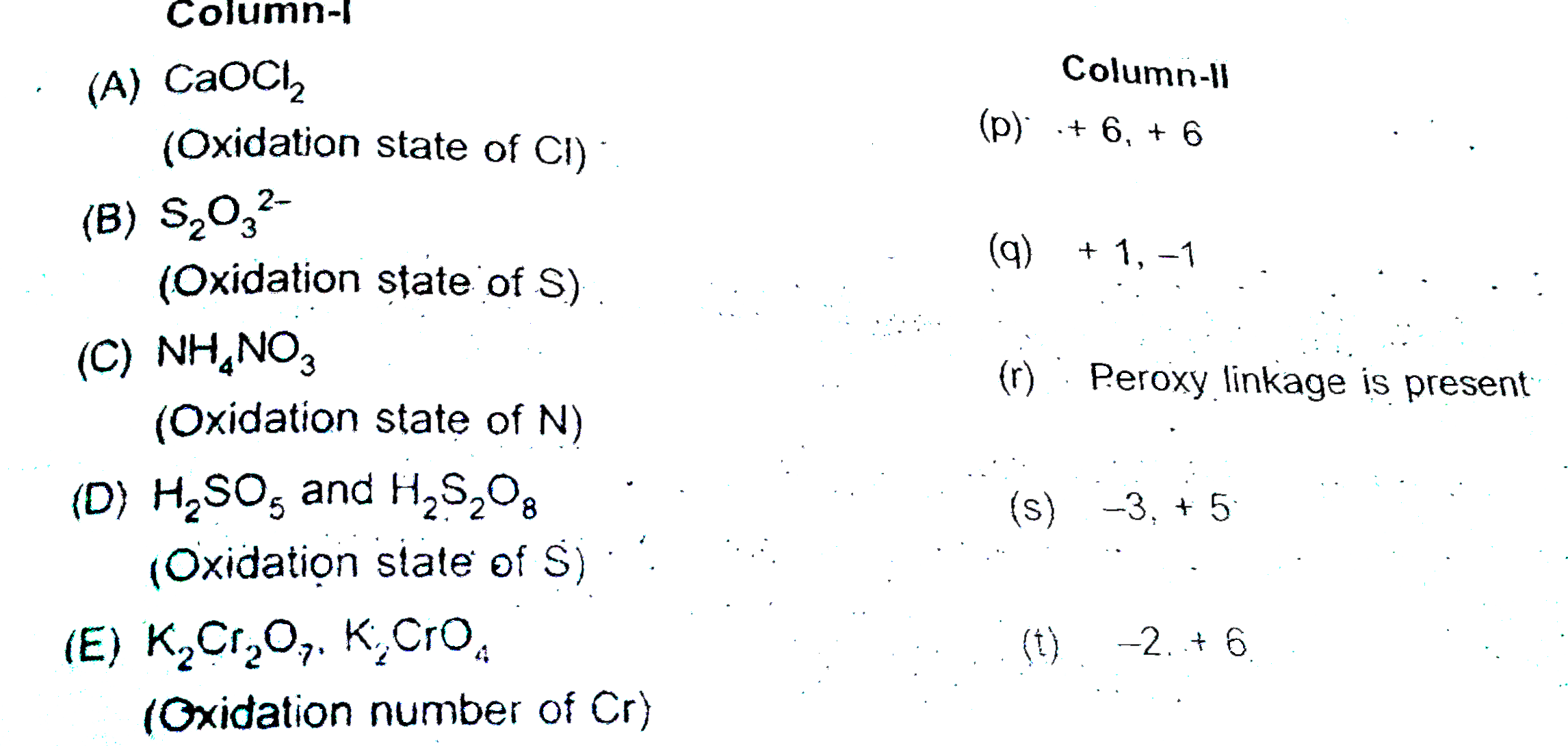
A(q),B(t),C(s),D(p,r),E(p)
Topper's Solved these Questions
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section G) (Interger Answer Type Questions)
Explore 8 Videos
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section H) (Multiple True-False Type Questions)
Explore 5 Videos
- REDOX REACTIONSBook:AAKASH INSTITUTE ENGLISHChapter:REDOX REACTIONSExercise:Assignment (Section E) (Assertion-Reason Type Questions)
Explore 8 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBook:AAKASH INSTITUTE ENGLISHChapter:PRINCIPLES OF QUALITATIVE ANALYSISExercise:Assignment (SECTION H)
Explore 9 Videos
- SOLUTIONSBook:AAKASH INSTITUTE ENGLISHChapter:SOLUTIONSExercise:ASSIGMENT (SECTION-J) AAKASH CHALLENGERS QUESTIONS
Explore 10 Videos
Similar Questions
0.144 g of pure FeC2O4 was dissolvedin dilute H2SO4 and the solution was diluted to 100 ml . What volume in ml of 0.1 M KMnO4 will be needed to oxidise FeC2O4 solution
View SolutionSTATEMENT-1 KMnO4 acts as powerful oxidising agent in acids, alkaline and neutral medium.
STATEMENT-2: Equivalent weight of KMnO4 in acidic medium in M/5 and in strongly alkaline medium in M/3.
STATEMENT-3 : KMnO4 solution is used as a primary standard and acts as self indicator during its titration more Mohr salt.
View SolutionSTATEMENT-1 KMnO4acidic medium−−−−−−−→Mn2+, n factor of KMnO4 is 5 and
STATEMENT-2 Equivalent mass of KMnO4 in acidic medium is M5 (M=molecular mass of KMnO4)
View SolutionWhen a white powder (A) is strongly heated, it gives of a colourless, odourless gas (B) which turns lime water milky (C) and if the passage of this gas is continued the milkiness disappears and gives a solution (D). The solid residue (E) is yellow when hot, but turns white on cooling. Idnetify (A) to (E) with help of the equations.
View SolutionAn aqueous solution of H2SO4 has density 1.84 g/ml. Solution contains 98% H2SO4 by mass.Calculate
(i) Molarity of solution
(ii) Molar volume of solution
(iii) Relative lowering of vapour pressure w.r.t. water, assuming H2SO4 as non-electrolyte at this high concentration.
View SolutionMatch the column I with the column II

View Solution
Match the column-I with Column-II
 .
.
View SolutionMatch the Column-I with Column-II
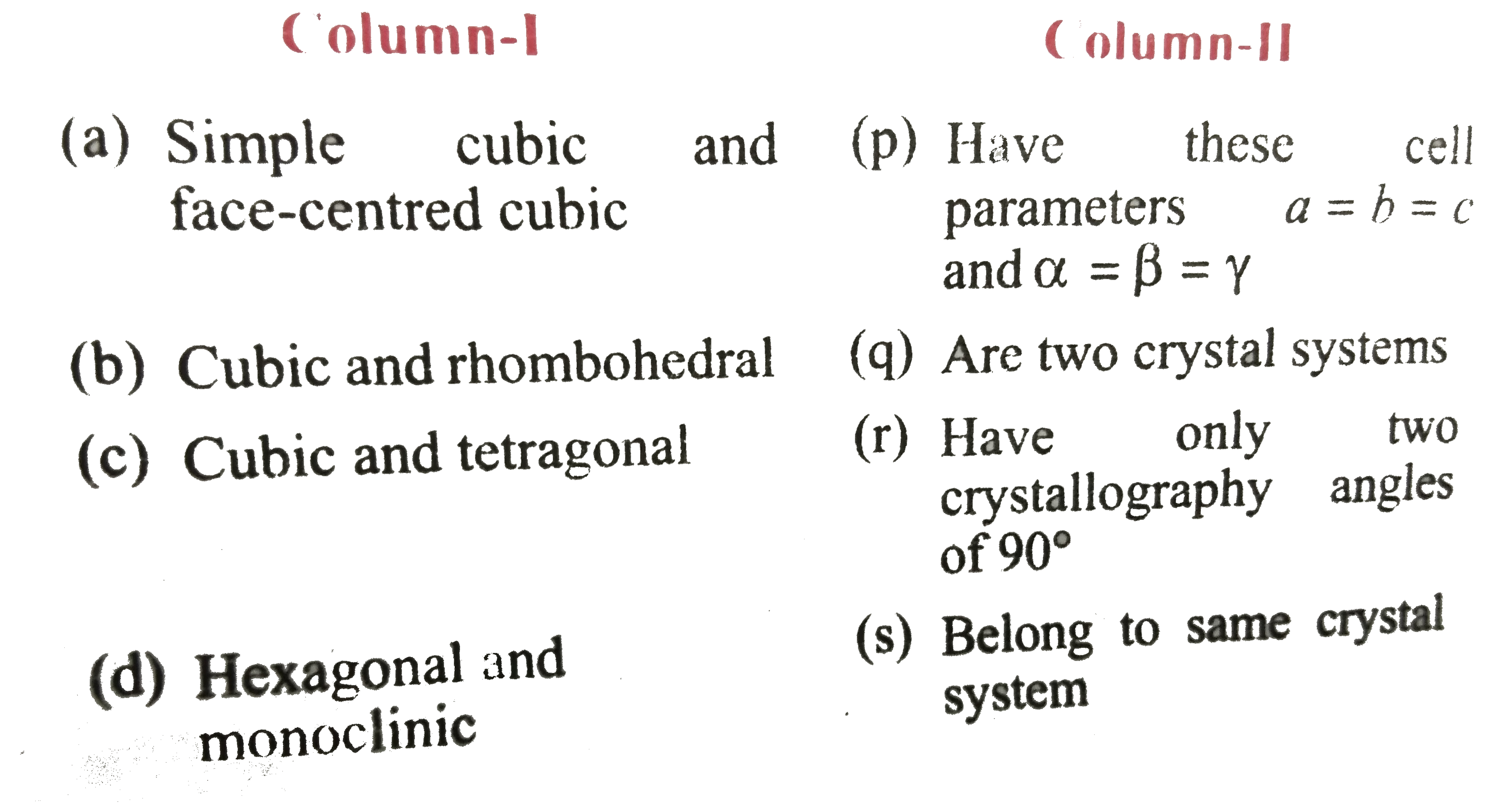
View SolutionMatche the Column-I with Column-II
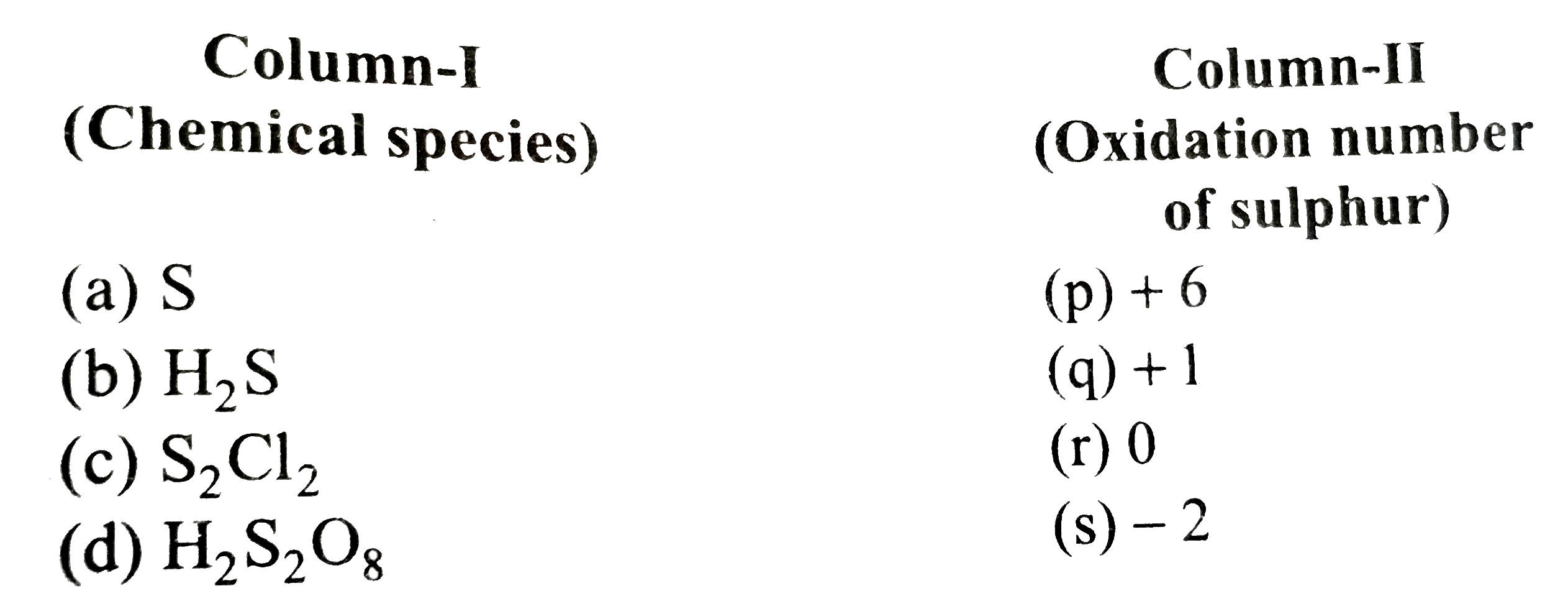
View SolutionMatche the Column-I with Column-II
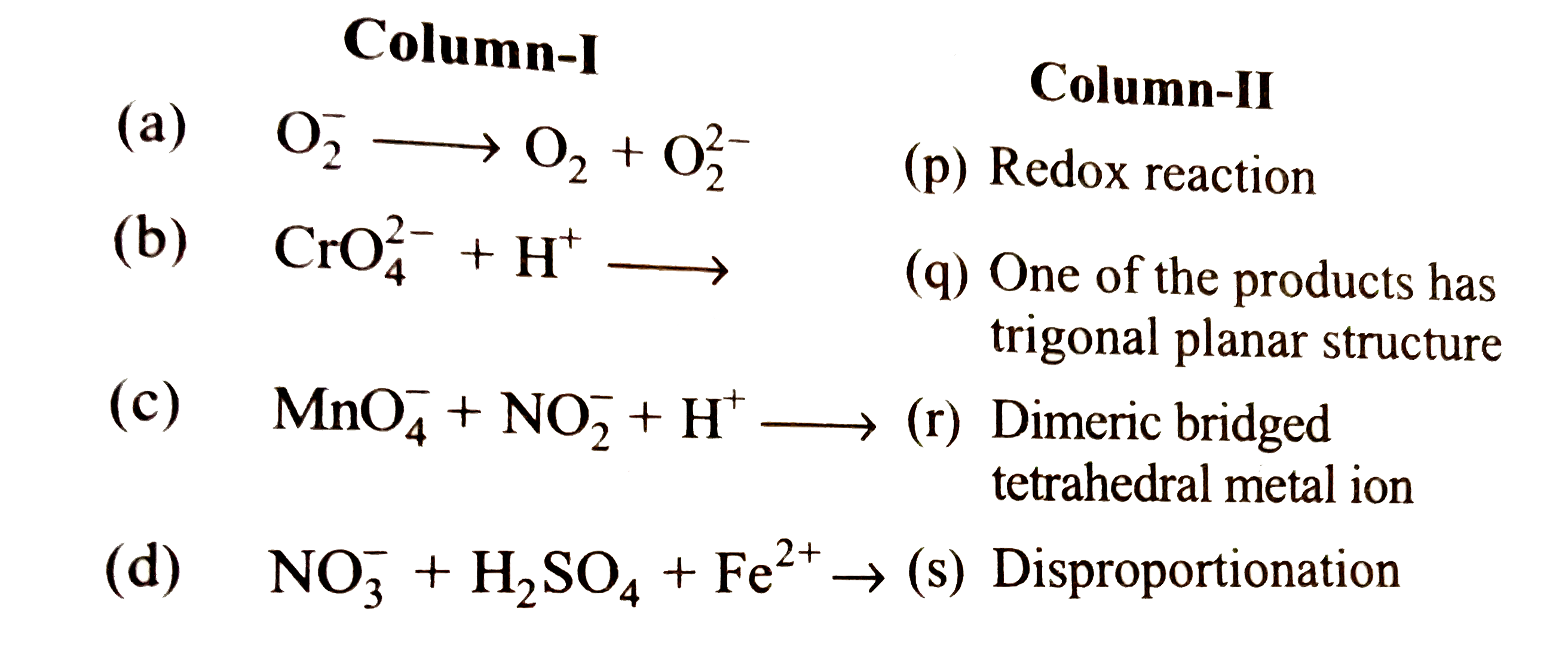
View SolutionMatch the column-I with Column-II

View SolutionMatch the column-I to column-II:-
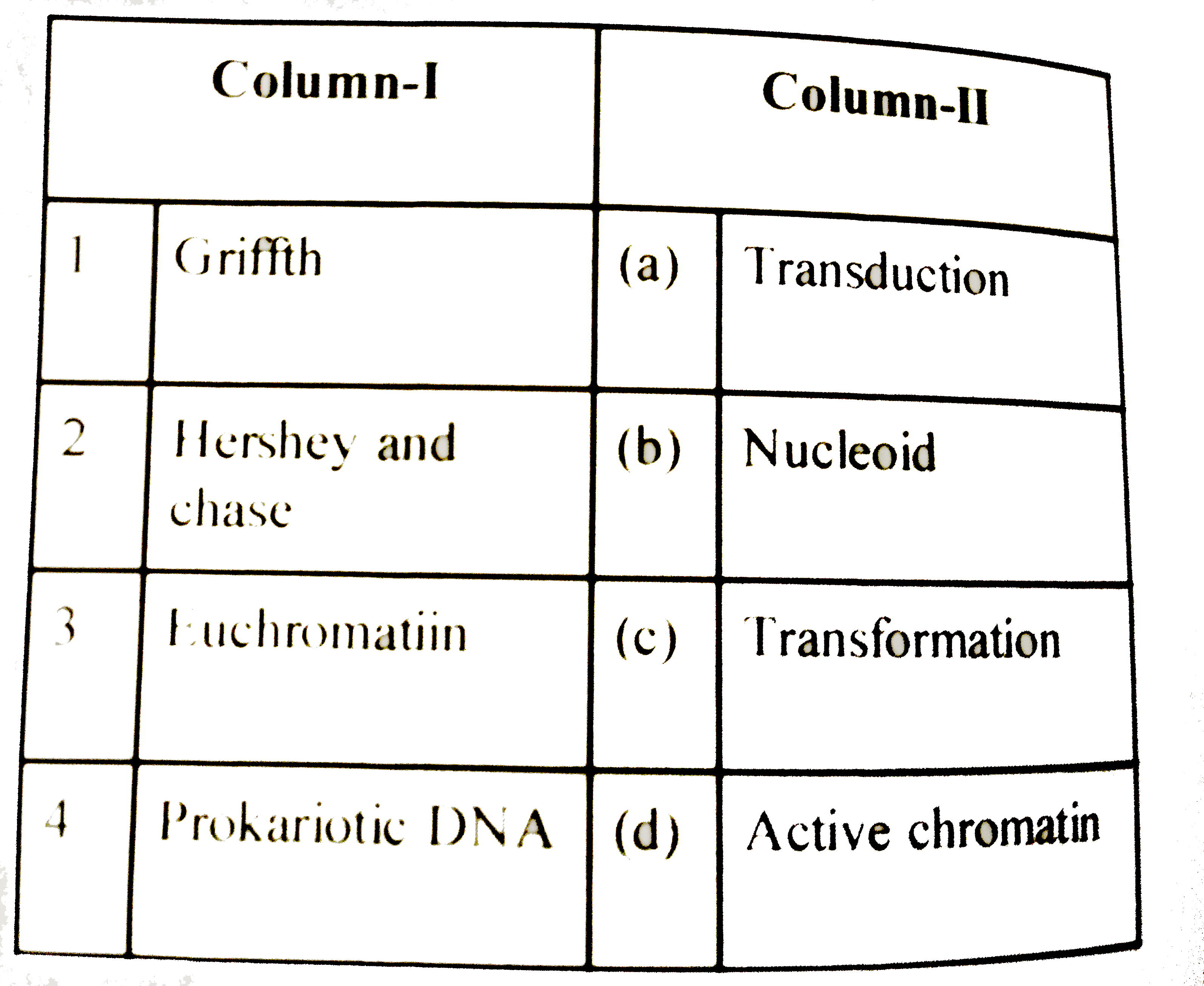
View SolutionMatch the column-I and Column-II
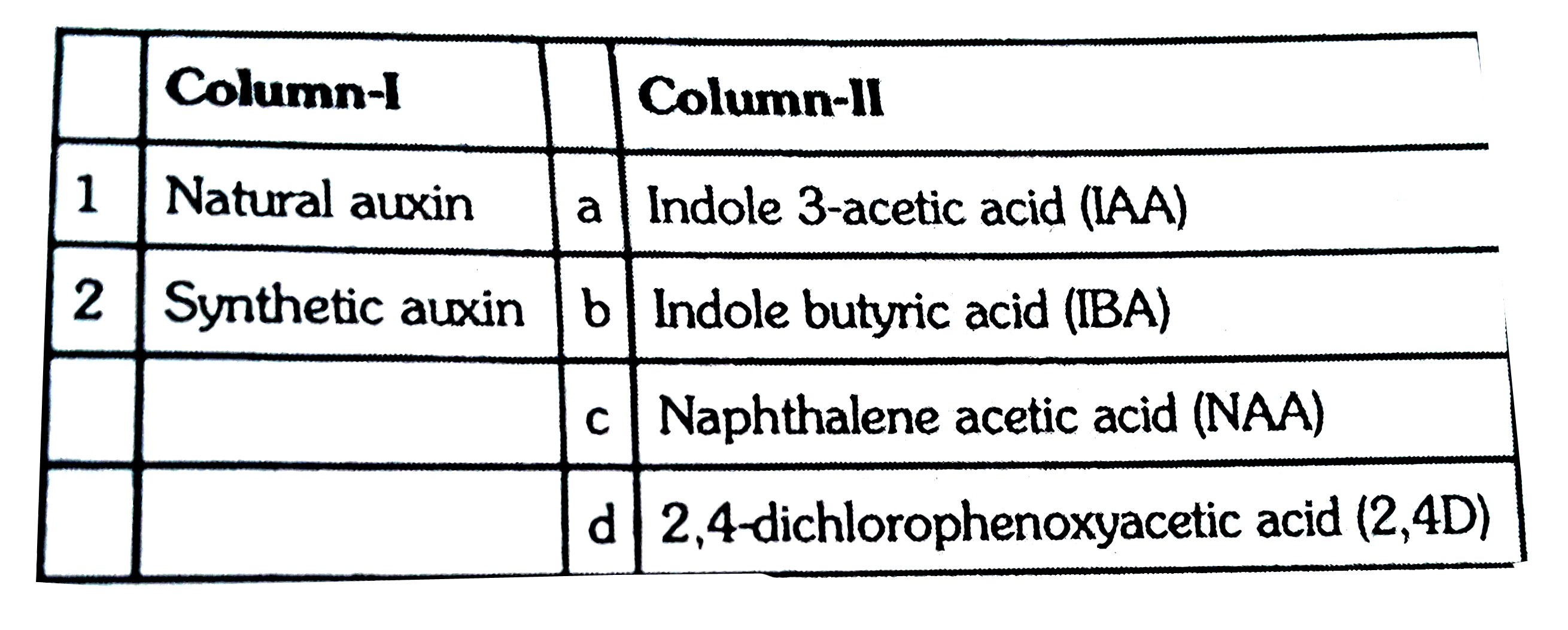
View SolutionMatch the column-I and column-II
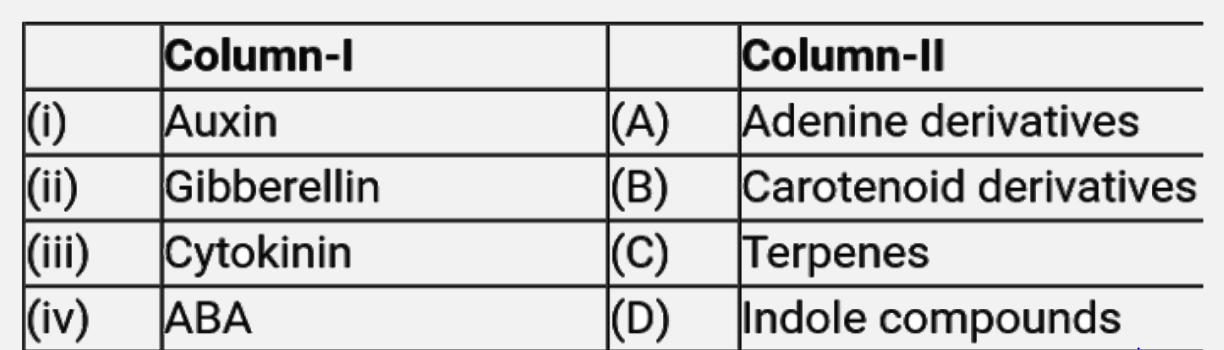
View SolutionMatch the Column -I with Column -II:

View SolutionMatch the Column -I with Column -II:

View Solution
