Statement-1:Amorphous solids are isotropic
Statement-2:Amorphous solids show physical properties like mechanical strength, electrical conductivity refractive index etc. same in all directions .
Statement-1 is true, statement-2 is true, statement-2 is a correct explanation for statement-1
Statement-1 is true, statement-2 is true, statement-2 is not correct explanation for statement-1
Statement-1 is true, statement-2 is false
Statement-1 is false, statement-2 is true
The correct Answer is:A
To analyze the given statements about amorphous solids, let's break down the reasoning step by step:
Step 1: Understand the concept of isotropy
- Isotropy refers to the property of a material where its physical properties are the same in all directions. This means that if you measure a property (like strength or conductivity) in any direction, you will get the same value.
Hint: Recall that isotropic materials have uniform properties regardless of the direction of measurement.
Step 2: Analyze Statement 1
- Statement 1: "Amorphous solids are isotropic."
- Amorphous solids, such as glass or certain plastics, lack a long-range order in their atomic arrangement. This irregular structure leads to uniformity in their physical properties in all directions.
Hint: Think about how the lack of a structured arrangement in amorphous solids contributes to their isotropic nature.
Step 3: Analyze Statement 2
- Statement 2: "Amorphous solids show physical properties like mechanical strength, electrical conductivity, refractive index, etc., the same in all directions."
- Due to the short-range order and random arrangement of particles in amorphous solids, their physical properties do not vary with direction. This means that mechanical strength, electrical conductivity, and refractive index are consistent regardless of the direction in which they are measured.
Hint: Consider how the random arrangement of atoms in amorphous solids affects their physical properties.
Step 4: Conclusion
- Since both statements are true and the second statement provides a correct explanation for the first, we conclude that:
- Both Statement 1 and Statement 2 are true.
- Statement 2 correctly explains why Statement 1 is true.
Final Answer: Both statements are true, and Statement 2 is the correct explanation for Statement 1.
- Isotropy refers to the property of a material where its physical properties are the same in all directions. This means that if you measure a property (like strength or conductivity) in any direction, you will get the same value.
- Statement 1: "Amorphous solids are isotropic."
- Amorphous solids, such as glass or certain plastics, lack a long-range order in their atomic arrangement. This irregular structure leads to uniformity in their physical properties in all directions.
- Statement 2: "Amorphous solids show physical properties like mechanical strength, electrical conductivity, refractive index, etc., the same in all directions."
- Due to the short-range order and random arrangement of particles in amorphous solids, their physical properties do not vary with direction. This means that mechanical strength, electrical conductivity, and refractive index are consistent regardless of the direction in which they are measured.
- Since both statements are true and the second statement provides a correct explanation for the first, we conclude that:
- Both Statement 1 and Statement 2 are true.
- Statement 2 correctly explains why Statement 1 is true.
Topper's Solved these Questions
- THE SOLID STATE Book:AAKASH INSTITUTE ENGLISHChapter:THE SOLID STATE Exercise:ASSIGNMENT SECTION-F (MATRIX MATCH)
Explore 2 Videos
- THE SOLID STATE Book:AAKASH INSTITUTE ENGLISHChapter:THE SOLID STATE Exercise:ASSIGNMENT SECTION-G (INTEGER)
Explore 6 Videos
- THE SOLID STATE Book:AAKASH INSTITUTE ENGLISHChapter:THE SOLID STATE Exercise:ASSIGNMENT SECTION-D (LINKED COMPREHENSION)
Explore 9 Videos
- THE S-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE S-BLOCK ELEMENTSExercise:Assignment (Section-J)
Explore 10 Videos
- THERMODYNAMICSBook:AAKASH INSTITUTE ENGLISHChapter:THERMODYNAMICSExercise:ASSIGNMENT (Section -D) Assertion-Reason Type Questions
Explore 15 Videos
Similar Questions
Match the following
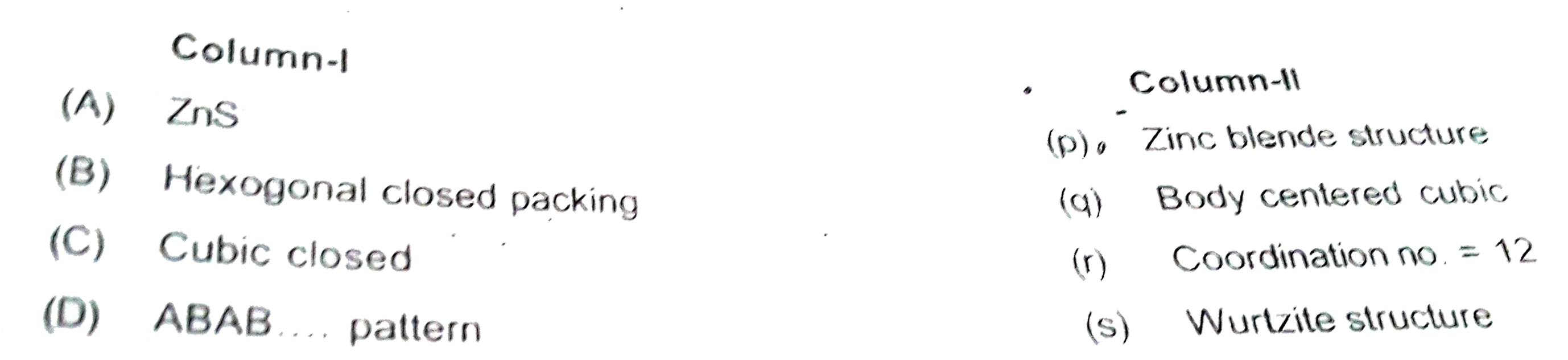
View SolutionWhat is the total no of planes of symmetry in a cube ?
View SolutionNaCl is a AB type of solid . Its crystalline structure is known as rock salt structure in which r+Na and r−Cl are 95 and 181 pm respectively , where Cl− is present at the lattice point of face centered cubic unit cell Na+ ions are located in void and those compounds which have same crystalline structure also have same coordination system in which Na+ and Cl− ions are surrounded by certain number of opposite ions respectively. Under high pressure coordination number changes to 8 :8 type from 6 :6 type.
On Subjecting NaCl under high pressure it changes
View SolutionChoose the correct statements among the following :
View SolutionA : Cdiamond→Cgraphite
ΔHandΔU are same for this reaction.
R: Entropy increases during the conversion of diamond to graphite.
View SolutionThe physical property (ies) which (is) are same along all the directions in an isotropic solid-
View SolutionSTATEMENT -1 : Amorphous solids are isotrophic STATEMENT -2 : Amorphous solids lack a regular three-dimensional arrangement of atoms.
View SolutionStatement-1:Amorphous solids are isotropic Statement-2:Amorphous solids show physical properties like mechanical strength, electrical conductivity refractive index etc. same in all directions .
View Solutionक्रिस्टलीय एवं अक्रिस्टलीय ठोसों मे से किसकी प्रकृति सैमदैशिक होती है ?
View SolutionA : Amorphous solids are isotropic.
R : Amorphous solids are not rigid.
View SolutionConsider the following statements. (i) Amorphous solids are isotropic like liquids (ii) Amorphous solids are considered as pseudo solids (iii) Amorphous solids have sharp melting points Which of the above statemetns is/are correct ?
View SolutionAssertion (A) : Amorphous solids are isotropic in nature.
Reason (R) : In amorphous solids, they have identical values of physical properties such as
refractive index, electrical conductance in all directions which is called isotropy.
View SolutionAmorphous solids that exhibit same physical properties in all the directions are called ---------.
View SolutionAmorphous solids that exhibit same physical properties in all the directions are called ---------.
View SolutionAmorphous solids that exhibit same physical properties in all the directions are called ---------.
View Solution
