A solid compound X on heating gives CO2 gas and a residue. The residue mixed with water forms Y. On passing an excess of CO2 through Y in water, a clear solution Z is obtained. On boiling Z, compound X is reformed, The compound X is
CaCO3
Na2CO3
Na(HCO3)2
K2CO3
The correct Answer is:A
Step by step video solution for A solid compound X on heating gives CO_(2) gas and a residue. The residue mixed with water forms Y. On passing an excess of CO_(2) through Y in water, a clear solution Z is obtained. On boiling Z, compound X is reformed, The compound X is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THE S-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE S-BLOCK ELEMENTSExercise:Assignment (Section-C)
Explore 11 Videos
- THE S-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE S-BLOCK ELEMENTSExercise:Assignment (Section-D)
Explore 9 Videos
- THE S-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE S-BLOCK ELEMENTSExercise:Assignment (Section-A)
Explore 33 Videos
- THE P-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE P-BLOCK ELEMENTSExercise:Assignment Section-J)
Explore 16 Videos
- THE SOLID STATE Book:AAKASH INSTITUTE ENGLISHChapter:THE SOLID STATE Exercise:Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)
Explore 20 Videos
Similar Questions
A compound (A) on heating in Busen flame imparts brick red colouration. (A) on heating gives CO2 gas and a residue (B). The residue (B) when treated with water gives (c). On passing an excess of CO2 through (C) in water, a clear solution (D) is obtained. On boiling (D), compound (A) is reformed.
Compound (A) is
View SolutionA compound (A) on heating in Busen flame imparts brick red colouration. (A) on heating gives CO2 gas and a residue (B). The residue (B) when treated with water gives (c). On passing an excess of CO2 through (C) in water, a clear solution (D) is obtained. On boiling (D), compound (A) is reformed.
Compound (C) is
View Solution
Knowledge Check
An element X burns in nitrogen to give a compound Y which on reaction with water gives a compound Z and a gas with a pungent smell. Z can be used during construction and white washing. When excess of CO2 is bubbled through Z, a compound P is formed which on heeating decomposes to give a compourless, odourless gas. identify X, Y, Z and P.
AX−Ca,Y−Ca3N2,Z−Ca(OH)2,P−Ca(HCO3)2BX−Mg,Y−MgO,Z−Mg(OH)2,P−MgCO3CX−Ca,Y−Ca3N2,Z−Ca(OH)2,P−CaCO3DX−Ca,Y−CaO,Z−Ca(OH)2,P−Ca(HCO3)2The order of stability of compounds X, Y and Z :
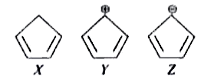 AX>Y>ZBY>Z>XCZ>X>YDX>Z>Y
AX>Y>ZBY>Z>XCZ>X>YDX>Z>Y
A compound (A) on heating in Busen flame imparts brick red colouration. (A) on heating gives CO2 gas and a residue (B). The residue (B) when treated with water gives (c). On passing an excess of CO2 through (C) in water, a clear solution (D) is obtained. On boiling (D), compound (A) is reformed.
Compound (D) is
View SolutionA compound (A) on heating in Busen flame imparts brick red colouration. (A) on heating gives CO2 gas and a residue (B). The residue (B) when treated with water gives (c). On passing an excess of CO2 through (C) in water, a clear solution (D) is obtained. On boiling (D), compound (A) is reformed.
Residue (B) is
View SolutionA compound X on heating gives a colourless gas. This residue is dissolved in water to obtain Y. excess CO2 is bubbled through aqueous solution of Y, Z is formed. Z on gentle heating give back X. the X is
View SolutionA compound X on heating gives a colourless gas. This residue is dissolved in water to obtain Y. excess CO2 is bubbled through aqueous solution of Y, Z is formed. Z on gentle heating give back X. the X is
View SolutionThe compound A on heating gives a colourless gas and a residue that
dissolved in water to obtain B. Excess of CO2 is bubbled through aqueous solution of B,C is formed which is recovered in the solid form.
Solid C on gentle heating gives back A. The compound is:-
View SolutionWhen an organic compound X is warmed with alkaline solution of KMnO4 a new compound Y is formed which smells of vinegar. On heating compounds X and Y with a few drops of concentrated sulphuric acid another compound Z is formed. Name the compound X and Y
View Solution
