Borazine is the product of reaction between
B2H6
NH3
BF3
BCl3
The correct Answer is:A, B
To determine the reaction that produces borazine, we need to analyze the given options and identify the correct reactants. Borazine is a compound with the formula B3H6N3.
1. Identify the Reactants: The question provides four options: B2H6 (diborane), NH3 (ammonia), BF3 (boron trifluoride), and BCl3 (boron trichloride). We need to find which two of these react to form borazine.
2. Understanding Borazine Formation: Borazine is known to be formed from the reaction of diborane (B2H6) and ammonia (NH3). This is a well-documented reaction in inorganic chemistry.
3. Write the Reaction: The reaction can be written as follows:
B2H6+6NH3→B3H6N3+6H2
This indicates that diborane reacts with ammonia to produce borazine and hydrogen gas.
4. Balancing the Reaction:
- On the left side, we have 2 boron atoms from B2H6 and 6 nitrogen atoms from NH3.
- On the right side, borazine contains 3 boron atoms and 3 nitrogen atoms.
- To balance the boron, we can adjust the coefficients:
3B2H6+6NH3→2B3H6N3+6H2
- This gives us the balanced equation.
5. Conclusion: From the analysis, we conclude that borazine is formed from the reaction between diborane (B2H6) and ammonia (NH3). Therefore, the correct answer is:
- B2H6 and NH3.
B2H6+6NH3→B3H6N3+6H2
This indicates that diborane reacts with ammonia to produce borazine and hydrogen gas.
- On the left side, we have 2 boron atoms from B2H6 and 6 nitrogen atoms from NH3.
- On the right side, borazine contains 3 boron atoms and 3 nitrogen atoms.
- To balance the boron, we can adjust the coefficients:
3B2H6+6NH3→2B3H6N3+6H2
- This gives us the balanced equation.
- B2H6 and NH3.
Topper's Solved these Questions
- THE P-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE P-BLOCK ELEMENTSExercise:Assignment Section-D)
Explore 15 Videos
- THE P-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE P-BLOCK ELEMENTSExercise:Assignment Section-E)
Explore 24 Videos
- THE P-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE P-BLOCK ELEMENTSExercise:Assignment Section-B)
Explore 26 Videos
- THE D AND F-BLOCK ELEMENTS Book:AAKASH INSTITUTE ENGLISHChapter:THE D AND F-BLOCK ELEMENTS Exercise:Assingment ( Section-J Aakash Challengers Questions )
Explore 10 Videos
- THE S-BLOCK ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:THE S-BLOCK ELEMENTSExercise:Assignment (Section-J)
Explore 10 Videos
Similar Questions
Knowledge Check
Which of the following, is the product of the reaction between AlCl3andCH3OCH3 ?
A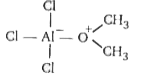 B
B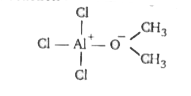 C
C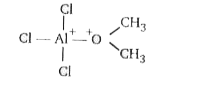 D
D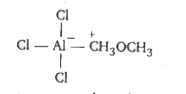
Bakelite is a product of reaction between
Aformaldehyde and NaOHBphenol and methanalCaniline and ureaDphenol and chloroform.Bakelite is a product of reaction between
Aformaldehyde and NaOHBphenol and methanalCaniline and ureaDphenol and chloroform.