An optically active compound [A] C5H13N reacts with alkaline CHCl3 to give an optically active, compound [B]. [A] also reacts with nitrous acid to give an optically inactive alcohol [C] C5H11OH as the major product. What would be the structure of [A]?
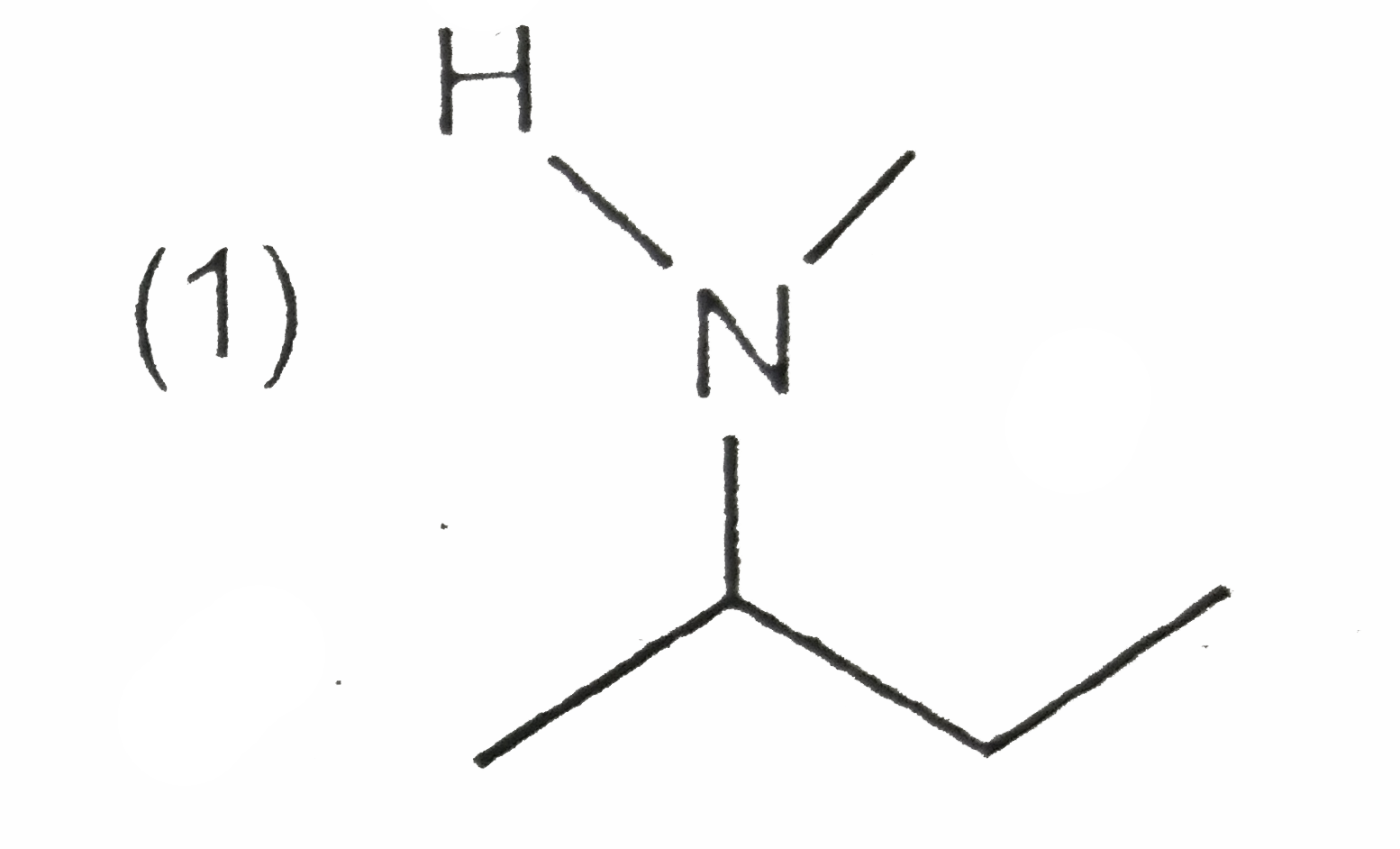
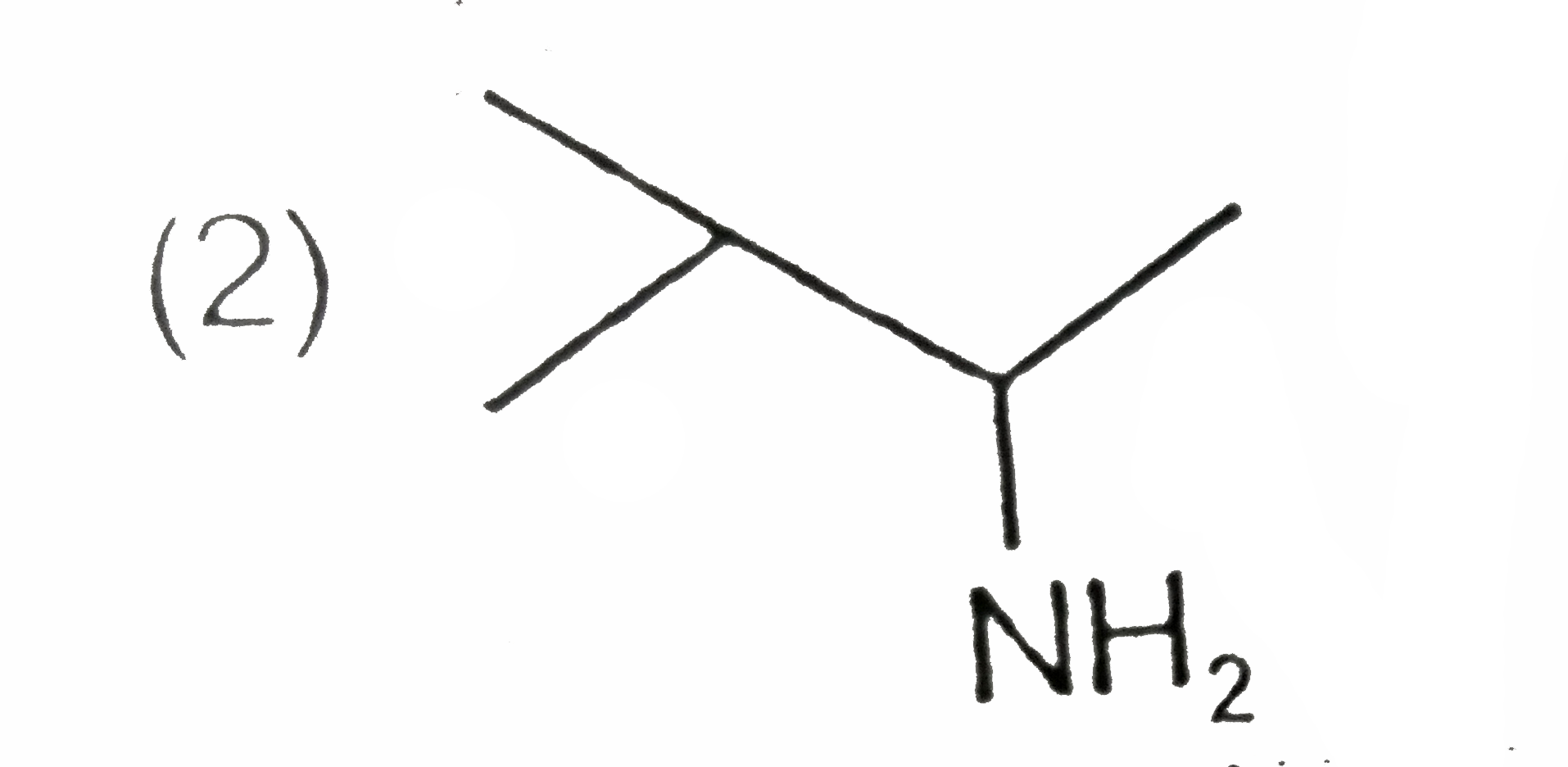
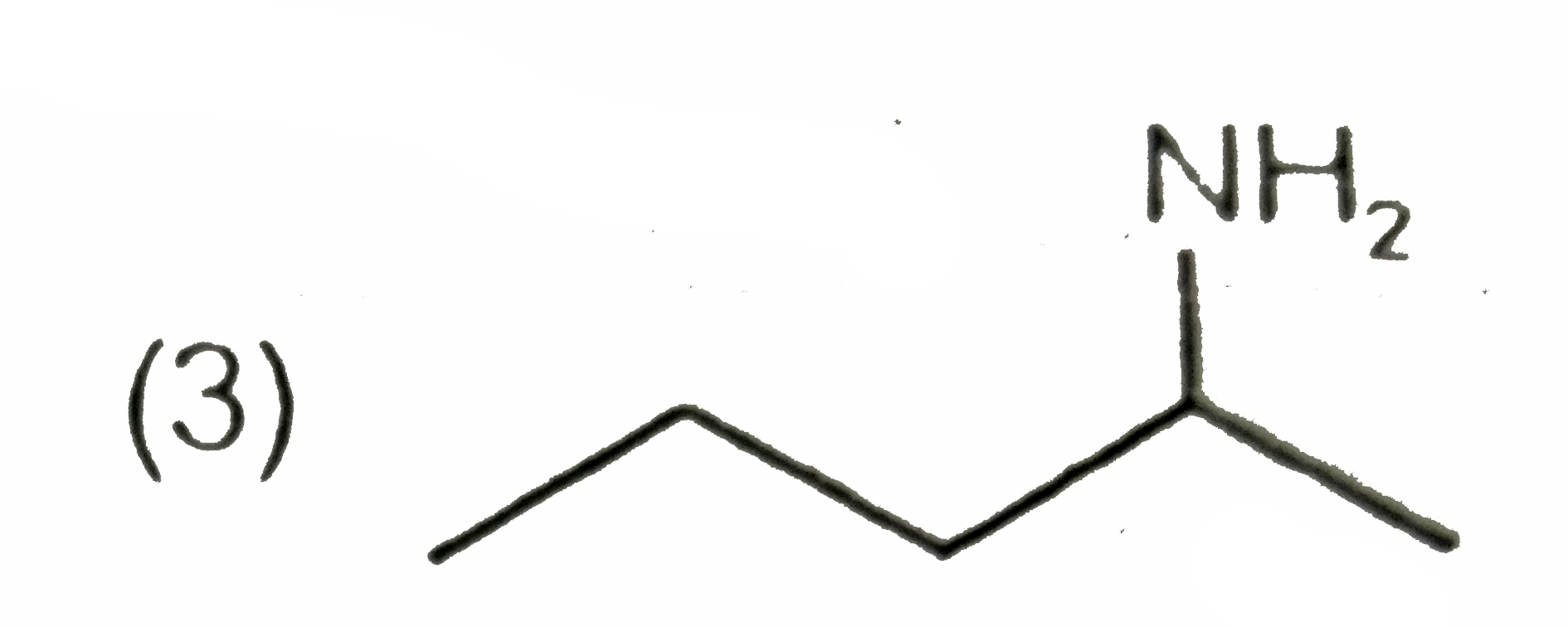

The correct Answer is:B
Step by step video solution for An optically active compound [A] C_(5)H_(13)N reacts with alkaline CHCl_(3) to give an optically active, compound [B]. [A] also reacts with nitrous acid to give an optically inactive alcohol [C] C_(5)H_(11)OH as the major product. What would be the structure of [A]? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- AMINES BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - AMINES EXERCISE - Assignment Section C (Objective type questions more than one options are correct)
12 Videos
- AMINES BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - AMINES EXERCISE - Assignment Section D Linked Comprehension Type Questions
15 Videos
- AMINES BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - AMINES EXERCISE - Assignment Section -A Competition Level Questions
50 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Try Yourself
20 Videos
- BIOMOLECULESBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - BIOMOLECULESEXERCISE - EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions
5 Videos
Similar Questions
Knowledge Check
Optically active isomer (A) of (C5H9Cl)on treatment with one mole of H2 gives an optically inactive compound (B) compound (A) will be :
ACH3−CH∣CH2Cl−CH=CH2BCl−CH∣CH3=CH−CH3CCH3−CH∣Cl−CH2−CH=CH2DCH3−CH2−CH∣Cl−CH=CH2Which of the following alkyne on treatment with H2 (2 mole)/Pt gives an optically inactive compound ?
A3-Methyl-1-pentyneB4-Methyl-1-hexyneC3-Methyl-1-heptyneDNone of the aboveCompound [X]C4H11N reacts with p− toluene sulphonyl chloride in aqueous NaOH to give a solid. The compound [X] is :
ACH3−CH2−CH2−CH2−NH2BH3C−C∣CH3H−CH2−NH2CCH3CH2−NH−CH2−CH3DH3C−CH2−N∣CH3−CH3
Racemic acid + optically active alcohol having chiral C atom →?
The product will be:
View SolutionTotal acyclic optically active isomers of C3H2D2 are :
View Solution(W) and (X) are optically active isomers of C5H9Cl (W) on treatment with 1mol of H2 is converted to an optically inactive compound (Y), but (X) gives an optically actgive compound (Z) under the same conditions. Give the sturcture of (Y) and the configurations of (W),(X) and (Z) in Fischer projections.
View SolutionPure enantiomeric acid+optically active alcohol having chiral C atom →?
The product will be:
View SolutionThe compound C5H13N is optically active and reacts with HONO to give C5H11OH. The compound is
View Solution

