Addition polymers are those in which monomers are converting into polymer by addltlon reaction. This addition reaction follow Radical mechanism in which firstly radical form than this propagate a chain than this chain terminate to form polymer
Which of the following is a propagation rejection?
.R+CH2=CH2→R−CH2−.CH2
RCH2CH2+CH2=CH2→R−CH2−CH2−CH2−.CH2
(RCH2CH2CH2.CH2)n→R(CH2CH2)2nR
None of these
The correct Answer is:B
To solve the question regarding which of the given reactions is a propagation rejection in the context of addition polymers formed through radical mechanisms, we can follow these steps:
1. Understand the Mechanism of Addition Polymers:
- Addition polymers are formed through a radical mechanism involving three main steps: initiation, propagation, and termination.
- In the initiation step, free radicals are generated.
- The propagation step involves the reaction of these free radicals with monomers, leading to the growth of the polymer chain.
- The termination step occurs when the growing polymer chain stops growing, resulting in the final polymer product.
2. Identify the Propagation Reaction:
- A propagation reaction involves the addition of a free radical to a monomer, resulting in a new radical that can continue to react with more monomers.
- The key characteristic of a propagation reaction is the presence of a free radical that continues the chain reaction.
3. Evaluate the Given Options:
- We need to analyze each of the provided reactions to determine if they involve a free radical and if they represent a propagation step.
- Look for reactions that do not involve the formation of a new radical or where the radical does not continue to react with additional monomers.
4. Determine the Correct Answer:
- After evaluating the reactions, identify which reaction does not fit the criteria of a propagation reaction. This will be the propagation rejection.
- In the provided options, the second reaction is identified as the one that does not involve a free radical in the propagation step, making it the correct answer.
5. Conclude:
- The correct option for the propagation rejection is the second reaction, as it does not involve the continuation of a radical chain.
Final Answer
The propagation rejection reaction is the second option.
- Addition polymers are formed through a radical mechanism involving three main steps: initiation, propagation, and termination.
- In the initiation step, free radicals are generated.
- The propagation step involves the reaction of these free radicals with monomers, leading to the growth of the polymer chain.
- The termination step occurs when the growing polymer chain stops growing, resulting in the final polymer product.
- A propagation reaction involves the addition of a free radical to a monomer, resulting in a new radical that can continue to react with more monomers.
- The key characteristic of a propagation reaction is the presence of a free radical that continues the chain reaction.
- We need to analyze each of the provided reactions to determine if they involve a free radical and if they represent a propagation step.
- Look for reactions that do not involve the formation of a new radical or where the radical does not continue to react with additional monomers.
- After evaluating the reactions, identify which reaction does not fit the criteria of a propagation reaction. This will be the propagation rejection.
- In the provided options, the second reaction is identified as the one that does not involve a free radical in the propagation step, making it the correct answer.
- The correct option for the propagation rejection is the second reaction, as it does not involve the continuation of a radical chain.
The propagation rejection reaction is the second option.
Topper's Solved these Questions
- POLYMERSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section- E (Assertion-Reason Type Questions )
3 Videos
- POLYMERSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section-F (Matrix match Type Questions )
6 Videos
- POLYMERSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section -C (Objective type Questions (More one option is correct ))
8 Videos
- ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESEXERCISE - TRY YOURSELF
28 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - PRINCIPLES OF QUALITATIVE ANALYSISEXERCISE - Assignment (SECTION H)
9 Videos
Similar Questions
Addition polymers are those in which monomers are converting into polymer by addltlon reaction. This addition reaction follow Radical mechanism in which firstly radical form than this propagate a chain than this chain terminate to form polymer
The polyethene is started as
View SolutionWhich of the following will undergo addition reactions ?
View Solution
Knowledge Check
Which of the following is a chain growth polymer?
AStarchBNucleic acidCPolystyreneDProteinWhich of the following is the free radical chain reaction?
A2CH3I+2Na→CH3−CH3+2NalBCH4+Cl2hv−−→CH3Cl+HClC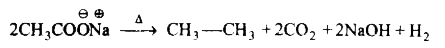 DAll of the above
DAll of the aboveWhich of the following is an addition polymer?
ANylonBMelamine formaldehyde resinCOrlonDPolystyrene
Which of the following is an addition polymer?
View SolutionWhich of the following is a free radical substitution reaction?
View SolutionWhich of the following is a chain-growth polymer?
View SolutionWhich of the following is a chain-growth polymer?
View SolutionWhich of the following is an addition polymer ?
View Solution

