In the Cannizzaro reaction given below:
2Ph−CHOΘOH−−→Ph−CH2OH+PhCO−2 the slowest step is:
The attack of OH− at the carbonyl group
The transfer of hydride to the carbonyl group
The abstraction of proton from the carboxylic group
The deprotonation of PhCH2OH
The correct Answer is:B
To determine the slowest step in the Cannizzaro reaction, let's break down the process step by step.
Step 1: Understanding the Cannizzaro Reaction
The Cannizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base. In this reaction, two molecules of an aldehyde (in this case, benzaldehyde) react to produce an alcohol and a carboxylate ion.
Hint: Recall that disproportionation reactions involve the simultaneous oxidation and reduction of the same species.
Step 2: Mechanism Overview
In the Cannizzaro reaction, one molecule of benzaldehyde is oxidized to form a carboxylate ion (benzoate), while the other molecule is reduced to form an alcohol (benzyl alcohol). The reaction requires a strong base, which facilitates the transfer of hydride ions.
Hint: Focus on how the aldehyde molecules interact with the base and each other during the reaction.
Step 3: Formation of the Anionic Intermediate
The reaction begins with the nucleophilic attack of hydroxide ion (OH⁻) on the carbonyl carbon of benzaldehyde. This forms an anionic intermediate where the oxygen carries a negative charge.
Hint: Identify the role of the hydroxide ion in initiating the reaction.
Step 4: Hydride Ion Transfer
The anionic intermediate formed can act as a reducing agent. It transfers a hydride ion (H⁻) to another molecule of benzaldehyde. This step is crucial, as it involves the transfer of a hydride ion to the electrophilic carbon of the second aldehyde molecule.
Hint: This is a key step where the reduction occurs; think about why this step might be slow.
Step 5: Rate Determining Step
The transfer of the hydride ion is the slowest step in the reaction mechanism and is known as the rate-determining step. This step controls the overall rate of the reaction, as it involves the breaking and forming of bonds.
Hint: Consider how the complexity of transferring a hydride ion affects the speed of the reaction.
Conclusion
In summary, the slowest step in the Cannizzaro reaction is the transfer of the hydride ion from the anionic intermediate to the carbonyl carbon of another benzaldehyde molecule. This step is critical as it determines the kinetics of the entire reaction.
Final Answer: The slowest step in the Cannizzaro reaction is the hydride ion transfer from the anionic intermediate to the carbonyl carbon of another aldehyde.
The Cannizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base. In this reaction, two molecules of an aldehyde (in this case, benzaldehyde) react to produce an alcohol and a carboxylate ion.
In the Cannizzaro reaction, one molecule of benzaldehyde is oxidized to form a carboxylate ion (benzoate), while the other molecule is reduced to form an alcohol (benzyl alcohol). The reaction requires a strong base, which facilitates the transfer of hydride ions.
The reaction begins with the nucleophilic attack of hydroxide ion (OH⁻) on the carbonyl carbon of benzaldehyde. This forms an anionic intermediate where the oxygen carries a negative charge.
The anionic intermediate formed can act as a reducing agent. It transfers a hydride ion (H⁻) to another molecule of benzaldehyde. This step is crucial, as it involves the transfer of a hydride ion to the electrophilic carbon of the second aldehyde molecule.
The transfer of the hydride ion is the slowest step in the reaction mechanism and is known as the rate-determining step. This step controls the overall rate of the reaction, as it involves the breaking and forming of bonds.
In summary, the slowest step in the Cannizzaro reaction is the transfer of the hydride ion from the anionic intermediate to the carbonyl carbon of another benzaldehyde molecule. This step is critical as it determines the kinetics of the entire reaction.
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (SECTION -B OBJECTIVE TYPES QUESTIONS (ONE OPTION IS CORRECT)
24 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (SECTION -C Objective type questions more than one options are correct)
11 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Illustrations
3 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - Try Yourself
5 Videos
- AMINES BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - AMINES EXERCISE - Assignment Section -J (Aakash Challengers Questions)
9 Videos
Similar Questions
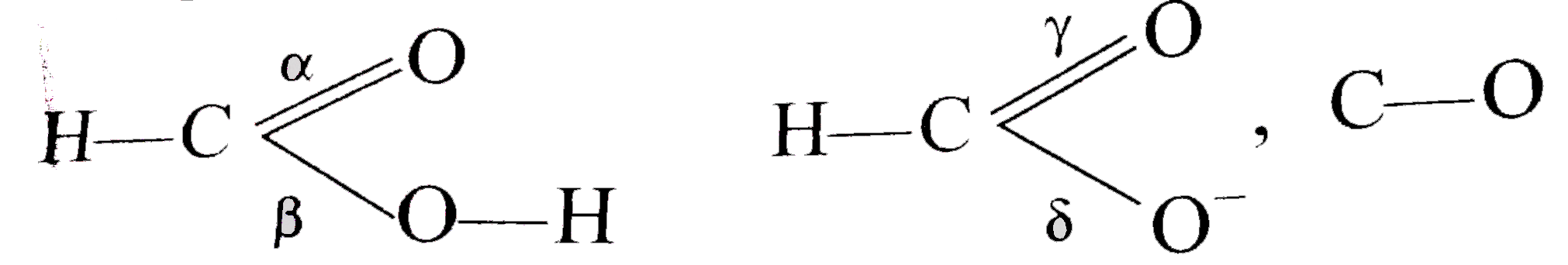 designated by α,β,γ and δ are in order:
designated by α,β,γ and δ are in order:
View SolutionWhich of the following reactions involve carbanion enolalte as reactive intermediate?
View SolutionPredict the major products of the following reactions:
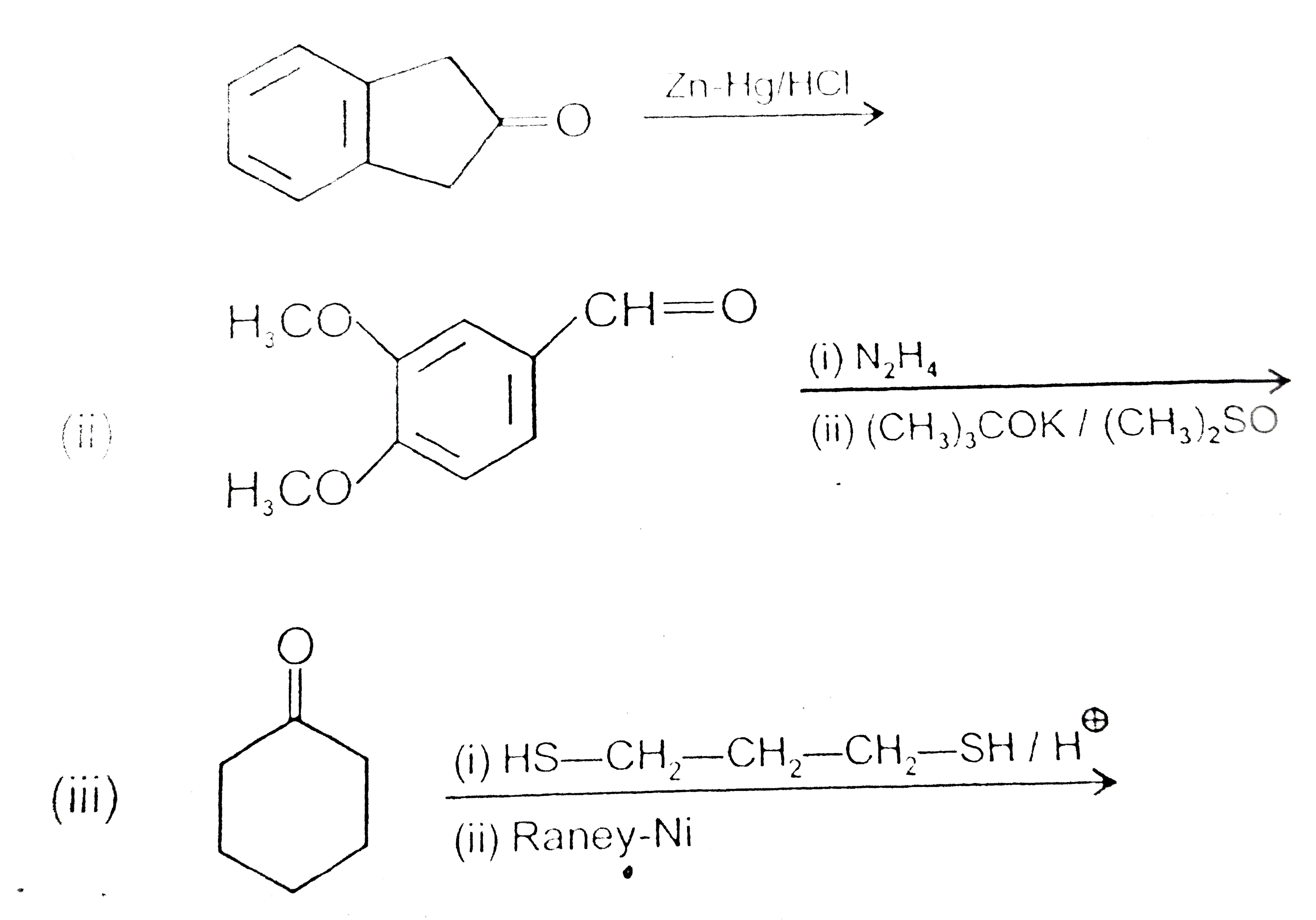
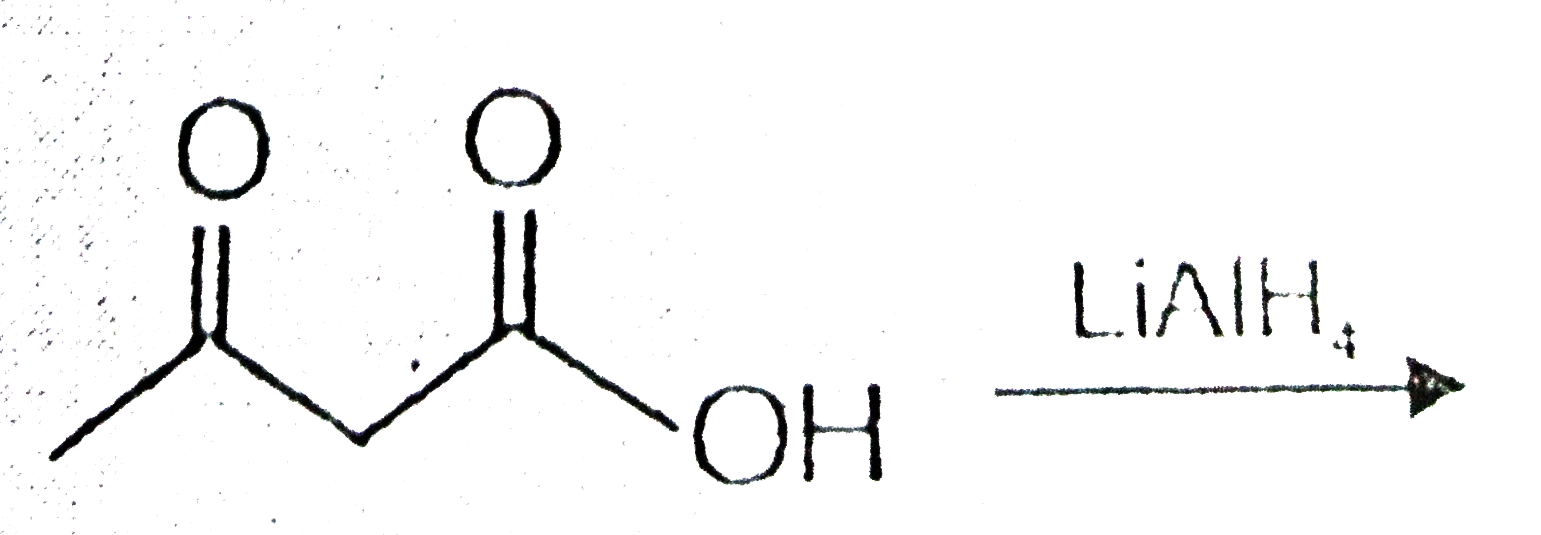
View SolutionFind the product of given reaction
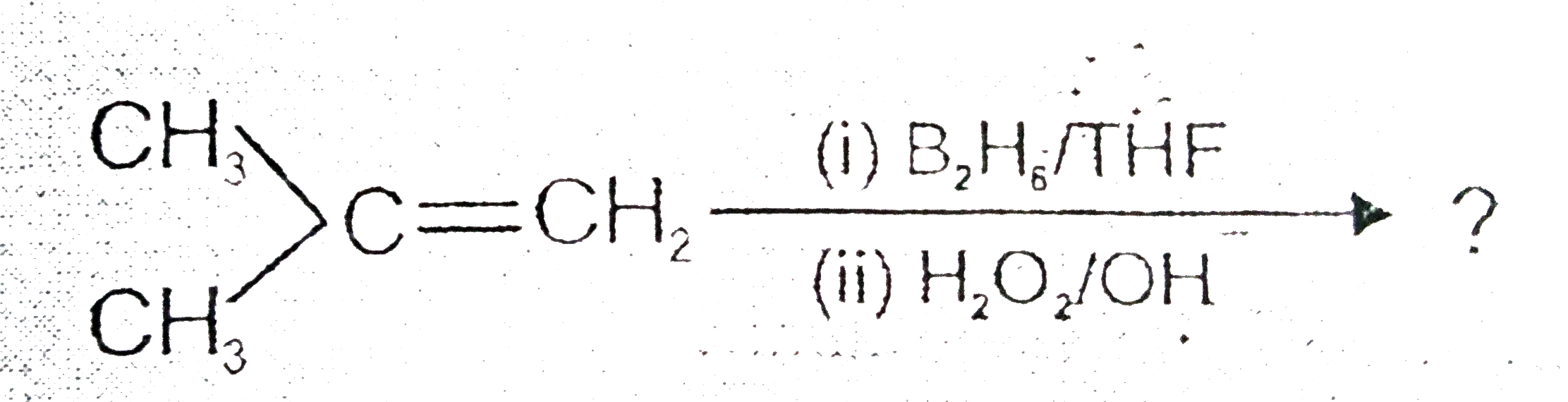
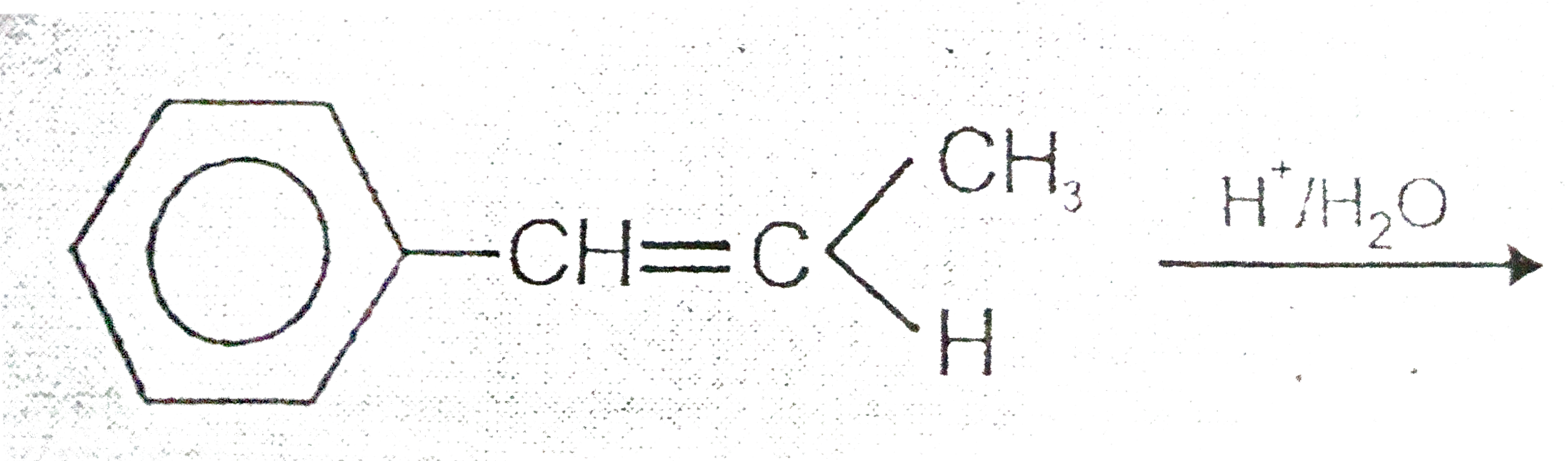

View SolutionFind intermediates and final product in the given sequence of reaction. Write mechanism for step-I and step- II.
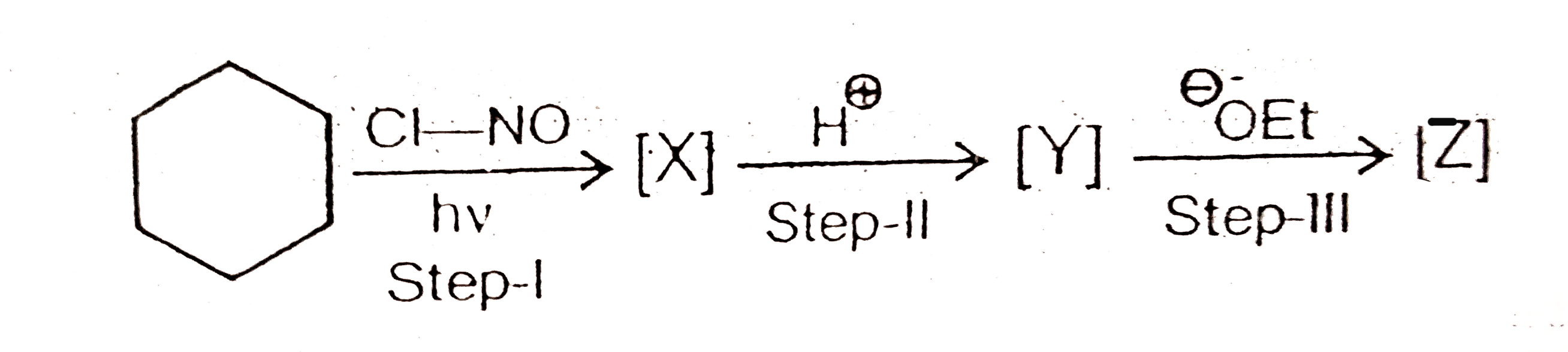
View SolutionWhich of the following is not the mechanism of Cannizzaro reaction ?
2PhCHOΘOH−−→PhCH2OH+PhCOOΘ
View SolutionIn the Cannizzaro reaction given below:
2Ph−CHOΘOH−−→Ph−CH2OH+PhCO−2 the slowest step is:
View SolutionConsider the following Cannizzaro reaction
2Ph−CHOOH−−−→Ph−CH2OH+PhCO−2
In the above reaction, the slowest step of the reaction is
View SolutionIn cannizzaro reaction given below 2PhCHO overset(":"overset(Theta)(O)H)toPhCH_(2)OH+Phddot(O)_(2)^(Theta) the slowest step is
View SolutionThe reaction given below is 2H - CHO overset(OH^(-)) to H - COONa + CH_(3) OH The slowest step is
View SolutionIn the Cannizzaro reaction given below:
2Ph−CHOΘOH−−→Ph−CH2OH+PhCO−2 the slowest step is:
View Solutionनीचे दी गई कैनिजारो अभिक्रिया में:
2Ph−CHOOH−−→Ph−CH2OH+PhCO2
सबसे मन्द पद है:
View Solution2PhCHOOHΘ−−→PhCH2OH+PhCOΘ2 कैनिजारो अभिक्रिया में मंद पद है-
View Solutionनीचे दी गई कैनिजारो अभिक्रिया में:
2Ph−CHOOH−−→Ph−CH2OH+PhCO2
सबसे मन्द पद है:
View SolutionIn the Cannizzaro reaction given below 2C_(6)H_(5)CHO overset(OH^(-))rarr C_(6)H_(5)CH_(2)OH+C_(6)H_(5)COO^(-) the slowest step is
View Solution

