The atmospheric pressure at a place iS 105 Pa. If tribromomethane (specific gravity = 2.9) be employed as the barometric liquid, the barometric height is:
3.52 m
1.52 m
4.52 m
2.52 m
To find the barometric height when using tribromomethane as the barometric liquid, we can follow these steps:
1. Understand the Given Data:
- Atmospheric pressure, P=105 Pa
- Specific gravity of tribromomethane, SG=2.9
2. Calculate the Density of Tribromomethane:
- The specific gravity (SG) is defined as the ratio of the density of the liquid to the density of water.
- The density of water, ρwater=1000kg/m3.
- Therefore, the density of tribromomethane, ρtribromomethane can be calculated as:
ρtribromomethane=SG×ρwater=2.9×1000kg/m3=2900kg/m3
3. Use the Hydrostatic Pressure Equation:
- The pressure exerted by a column of liquid is given by the formula:
P=ρgh
- Where:
- P is the pressure (in Pascals),
- ρ is the density of the liquid (in kg/m³),
- g is the acceleration due to gravity (approximately 9.8m/s2),
- h is the height of the liquid column (in meters).
4. Rearranging the Equation to Solve for Height h:
- We can rearrange the equation to solve for h:
h=Pρg
5. Substituting the Values:
- Now, substituting the known values into the equation:
h=105Pa2900kg/m3×9.8m/s2
6. Calculating the Height:
- Calculate the denominator:
2900×9.8=28420kg/(m2s2)
- Now, calculate h:
h=10528420≈3.52m
7. Conclusion:
- The barometric height of tribromomethane is approximately 3.52m.
Final Answer:
The barometric height is 3.52m.
---
- Atmospheric pressure, P=105 Pa
- Specific gravity of tribromomethane, SG=2.9
- The specific gravity (SG) is defined as the ratio of the density of the liquid to the density of water.
- The density of water, ρwater=1000kg/m3.
- Therefore, the density of tribromomethane, ρtribromomethane can be calculated as:
ρtribromomethane=SG×ρwater=2.9×1000kg/m3=2900kg/m3
- The pressure exerted by a column of liquid is given by the formula:
P=ρgh
- Where:
- P is the pressure (in Pascals),
- ρ is the density of the liquid (in kg/m³),
- g is the acceleration due to gravity (approximately 9.8m/s2),
- h is the height of the liquid column (in meters).
- We can rearrange the equation to solve for h:
h=Pρg
- Now, substituting the known values into the equation:
h=105Pa2900kg/m3×9.8m/s2
- Calculate the denominator:
2900×9.8=28420kg/(m2s2)
- Now, calculate h:
h=10528420≈3.52m
- The barometric height of tribromomethane is approximately 3.52m.
The barometric height is 3.52m.
Similar Questions
The atmospheric pressure and height of barometer column is 105Pe and 760 mm respectively on the earth surface. If the barometer is taken to the moon then column height will be-
View SolutionOxygen is filled in a closed metal jar of volume 1.0×10−3m3 at a pressure of 1.5×105Pa. and temperature 400K.The jar has a small leak in it. The atmospheric pressure is 1.0×105Pa and the atmospheric temperature is 300K. Find the mass of the gas that leaks out by time the pressure and the temperature inside the jar equlise with the surrounding.
View SolutionThe density of asir ner earth's surface is 1.3kgm−3 and the atmospheric pressure is 1.0×105Nm−2. If the atmosphere had uniform density, same as that observed at the surface of the earth what would be the height of the atmosphere to exert the same pressure?
View Solution10 mL of liquid carbon disulphide (specific gravity 2.63) is burnt in oxygen. Find the volume of the resulting gases measured at STP.
View SolutionAtmospheric pressures recorded in different cities are as follows :
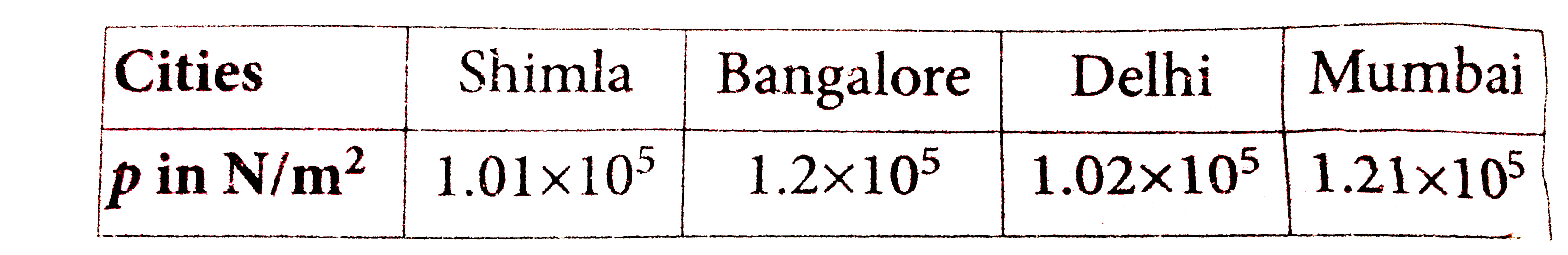
Consider the above data and mark the place at which liquid will boil first .
View SolutionFluids at rest exert a normal force to the walls of the container or to the sruface of the body immersed in the fluid. The pressure exerted by this force at a point inside the liqid is the sum of atmospheric pressure and a factor which depends on the density of the liquid, the acceleration due to gravity and the height of the liquid, above that point. The upthrust acting on a body immersed in a stationary liquid is the net force acting on the body in the upward direction. A number of phenomenon of liquids in motion can be explain by Bernoulli's theorem which relates the pressure, flow speed and height for flow of an ideal incompressible fluid.
A container of large uniform corss sectional area. A resting on a horizontal surface holds two immiscible, non viscous and incompressile liquids of densities d and 2d, each of height H/2 as shown in the figure. The lower density liquid is open to the atmosphere having pressure P0.
Situation I:
A homogeneous solid cylinder of length L(L<H/2). cross sectional area A/5 is immersed such that it floats with its axis vertical at liquid -liquid interface with lenght L/4 in the denser liquid.
The total pressure at the bottom of the container is
View SolutionWhat is meant by the statement “the atmospheric pressure at a place is 76 cm of Hg"? State its value in Pa.
View SolutionA vessel of base area 100cm×60cm and height 200cm is completely filled with a liquid of density 1.1×103kgm−3.
(a) Ignoring the atmospheric pressure, find :
(i) the thrust at the bottom of the vessel,
(ii) the pressure at the bottom of the vessel,
(iii) The pressure at a depth of 5 cm from the free surface,
(iv) the net force experienced by a metal foil of area 10cm2 placed at a depth of 5 cm from the free surface,
(b) The thrust at the bottom of the vessel if the atmospheric pressure equal to 1×105Nm−2 is taken into account.
Take g=9.8ms−2 .
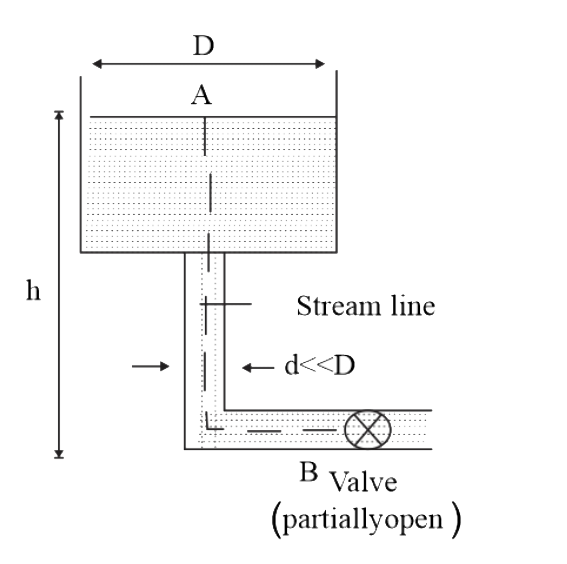
View SolutionA storage tower supplies water, as shown in the figure. If P0 is the atmospheric pressure h = height of water level, g = acceleration due to gravity, ρ= density and v = velocity of flow in the horizontal pipe at B, then the pressure at B is -
View SolutionAt sea level, the atmospheric pressure is 1.04×105 Pa. Assuming g=10m s−2 and density of air to be uniform and equal to 1.3kg m−3, find the height of the atmosphere.
View Solution
