When a gas undergoes adiabatic expansion, it gets cooles. This is because
It is an exothermic process
It is an endothermic process
Internal energy of the system decreases
Ideal gas becomes a real gas
The correct Answer is:C
To understand why a gas cools during adiabatic expansion, we can analyze the process step by step using the principles of thermodynamics.
1. Understanding Adiabatic Process:
- An adiabatic process is one in which no heat is exchanged with the surroundings. This means that the heat transfer (Q) is equal to zero.
- Hint: Remember that in an adiabatic process, the system is insulated from heat exchange.
2. First Law of Thermodynamics:
- The first law of thermodynamics states that the change in internal energy (ΔU) of a system is equal to the heat added to the system (Q) plus the work done on the system (W):
ΔU=Q+W
- Since Q = 0 for an adiabatic process, this simplifies to:
ΔU=W
- Hint: Keep in mind that work done on the system is considered positive, while work done by the system is negative.
3. Work Done During Expansion:
- During adiabatic expansion, the gas does work on the surroundings. Therefore, W is negative (since the gas is expanding).
- This means:
ΔU=W<0
- Hint: When a gas expands, it uses energy to do work, which leads to a decrease in internal energy.
4. Internal Energy and Temperature:
- The internal energy (U) of an ideal gas is directly related to its temperature. If the internal energy decreases (ΔU < 0), it implies that the temperature of the gas also decreases.
- Hint: Remember that for an ideal gas, a decrease in internal energy corresponds to a decrease in temperature.
5. Conclusion:
- Therefore, during adiabatic expansion, the gas cools down because its internal energy decreases as it does work on the surroundings without any heat being added to compensate for that energy loss.
- The correct answer to the question is that the internal energy of the system decreases (Option C).
Final Answer:
The gas cools during adiabatic expansion because the internal energy of the system decreases.
- An adiabatic process is one in which no heat is exchanged with the surroundings. This means that the heat transfer (Q) is equal to zero.
- Hint: Remember that in an adiabatic process, the system is insulated from heat exchange.
- The first law of thermodynamics states that the change in internal energy (ΔU) of a system is equal to the heat added to the system (Q) plus the work done on the system (W):
ΔU=Q+W
- Since Q = 0 for an adiabatic process, this simplifies to:
ΔU=W
- Hint: Keep in mind that work done on the system is considered positive, while work done by the system is negative.
- During adiabatic expansion, the gas does work on the surroundings. Therefore, W is negative (since the gas is expanding).
- This means:
ΔU=W<0
- Hint: When a gas expands, it uses energy to do work, which leads to a decrease in internal energy.
- The internal energy (U) of an ideal gas is directly related to its temperature. If the internal energy decreases (ΔU < 0), it implies that the temperature of the gas also decreases.
- Hint: Remember that for an ideal gas, a decrease in internal energy corresponds to a decrease in temperature.
- Therefore, during adiabatic expansion, the gas cools down because its internal energy decreases as it does work on the surroundings without any heat being added to compensate for that energy loss.
- The correct answer to the question is that the internal energy of the system decreases (Option C).
The gas cools during adiabatic expansion because the internal energy of the system decreases.
Topper's Solved these Questions
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - ASSIGNMENT (Section - A) Objective Type Questions
55 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - ASSIGNMENT (Section - B) Objective Type Questions
35 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - SECTION-J
7 Videos
- THE SOLID STATE BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THE SOLID STATE EXERCISE - Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)
20 Videos
Similar Questions
Tea placed in thermos flask is an example of
View SolutionLet (Cv)and(Cp) denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively . Which of the following is a universal constant?
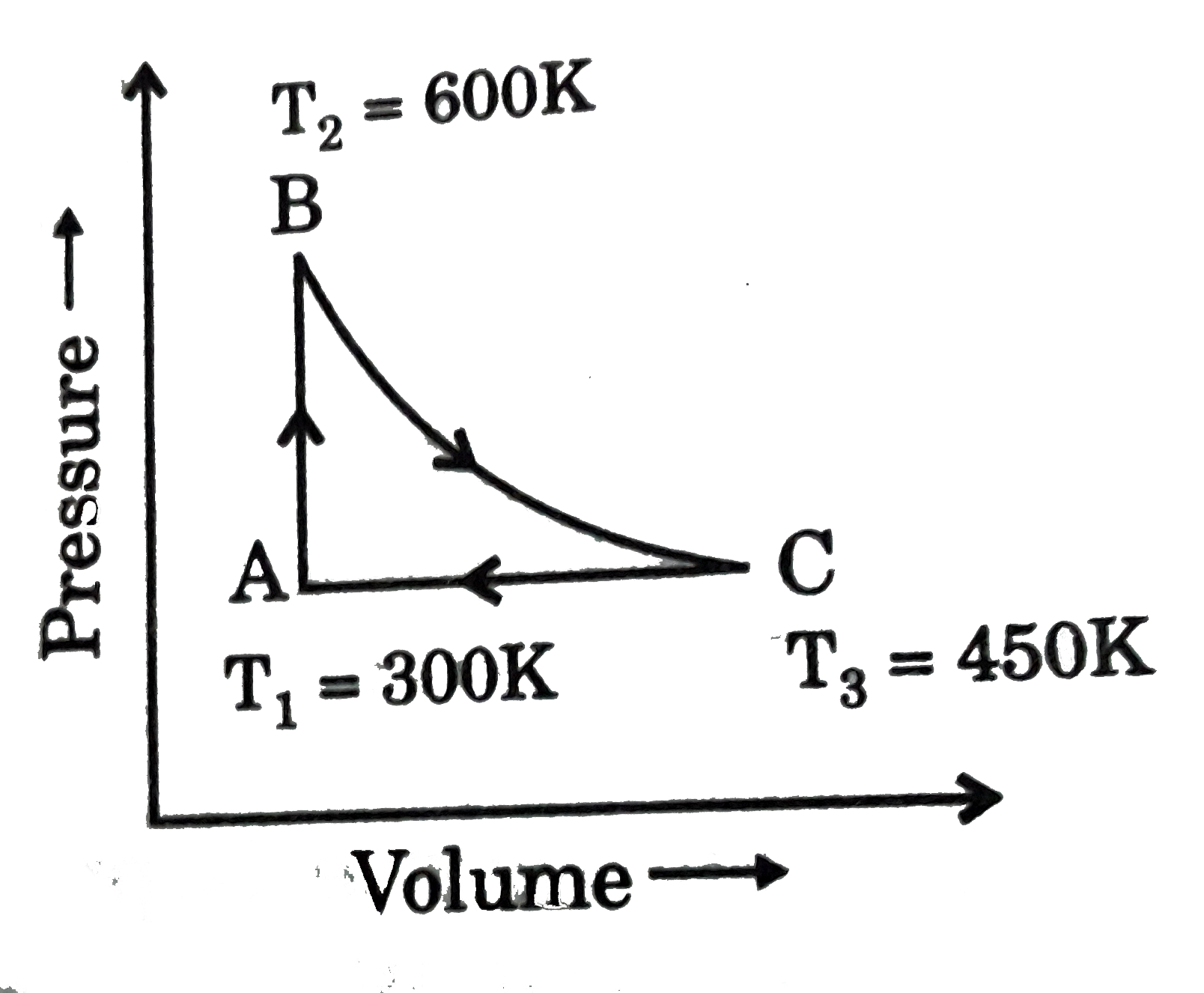
View SolutionA heat engine carries one mole of an ideal monoatomic gas around the cycle as shown in the figure, the amount of heat added in the process AB and heat removed in the process CA are :
View SolutionA : In NaCl structure, the interionic distance is a/2 (a = Unit cell edge length).
R : NaCl forms face centered cubic unit cell.
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen a gas is subjected to adiabatic expansion, it gets cooled due to :
View SolutionWhen a gas is subjected to adiabatic expansion, it gets cooled due to
View SolutionWhen a gas is subjected to adiabatic expansion, it gets cooled due to :
View SolutionWhen a gas is subjected to adiabatic expansion, it gets cooled due to
View SolutionWhen an iden gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen a gas undergoes adiabatic expansion, it gets cooles. This is because
View SolutionWhen an ideal gas undergoes unrestrained expansion, on cooling occurs because the molecules
View SolutionWhen an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules
View SolutionWhen an ideal gas undergoes undestrained expansion, no cooling occurs because the molecules
View SolutionA gas on subjecting to adiabatic expansion gets cooled due to:
View Solution

