C6H5N2+Cl−Cu2Cl2/HCl−−−−−−−−−→ , this reaction is named as
Sandmeyer
Swarts
Wurtz-fitting
Finkelstein
The correct Answer is:A
To determine the name of the reaction represented by the equation C6H5N+2Cl−Cu2Cl2/HCl→C6H5Cl, we will analyze the reaction step by step.
Step 1: Identify the Reactants
The reactants in the equation are:
- C6H5N+2: This is an aryl diazonium ion.
- Cl−: This is a chloride ion.
Hint: Aryl diazonium ions are often involved in reactions to form aryl halides.
Step 2: Identify the Reaction Conditions
The reaction occurs in the presence of:
- Cu2Cl2: Copper(I) chloride, which acts as a reagent to facilitate the substitution.
- HCl: Hydrochloric acid, which provides the chloride ions necessary for the reaction.
Hint: The presence of copper salts often indicates a coupling or substitution reaction involving diazonium compounds.
Step 3: Determine the Product
The product formed from this reaction is:
- C6H5Cl: This is chlorobenzene, an aryl halide.
Hint: Aryl diazonium salts typically react to form aryl halides when treated with halide ions in the presence of copper salts.
Step 4: Identify the Type of Reaction
This reaction is a classic example of the Sandmeyer reaction. In the Sandmeyer reaction, aryl diazonium salts are converted into aryl halides using copper(I) halides.
Hint: Look for reactions involving diazonium salts that lead to the formation of halogenated aryl compounds.
Conclusion
The reaction C6H5N+2Cl−Cu2Cl2/HCl→C6H5Cl is known as the Sandmeyer reaction.
Final Answer: Sandmeyer reaction.
The reactants in the equation are:
- C6H5N+2: This is an aryl diazonium ion.
- Cl−: This is a chloride ion.
The reaction occurs in the presence of:
- Cu2Cl2: Copper(I) chloride, which acts as a reagent to facilitate the substitution.
- HCl: Hydrochloric acid, which provides the chloride ions necessary for the reaction.
The product formed from this reaction is:
- C6H5Cl: This is chlorobenzene, an aryl halide.
This reaction is a classic example of the Sandmeyer reaction. In the Sandmeyer reaction, aryl diazonium salts are converted into aryl halides using copper(I) halides.
The reaction C6H5N+2Cl−Cu2Cl2/HCl→C6H5Cl is known as the Sandmeyer reaction.
Topper's Solved these Questions
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTE ENGLISHChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION - B
Explore 25 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTE ENGLISHChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION - C
Explore 39 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTE ENGLISHChapter:HALOALKANES AND HALOARENES Exercise:EXERCISE
Explore 40 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBook:AAKASH INSTITUTE ENGLISHChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSExercise:Try Yourself
Explore 33 Videos
- HYDROCARBONSBook:AAKASH INSTITUTE ENGLISHChapter:HYDROCARBONSExercise:Assignment(Section - C) (Previous Years Questions)
Explore 60 Videos
Similar Questions
Knowledge Check
The reaction Ar+N2Cl−Cu/HCl−−−−→ArCl+N2+CuCl is named as _____
ASandmeyer reactionBGatterman reactionCHofmann bromamie degradation reactionDCarbylamine reaction
Write the main products of the following reactions:
(i) C6H5N+2Cl−H3PO2+H2O−−−−−−−−→?
(ii)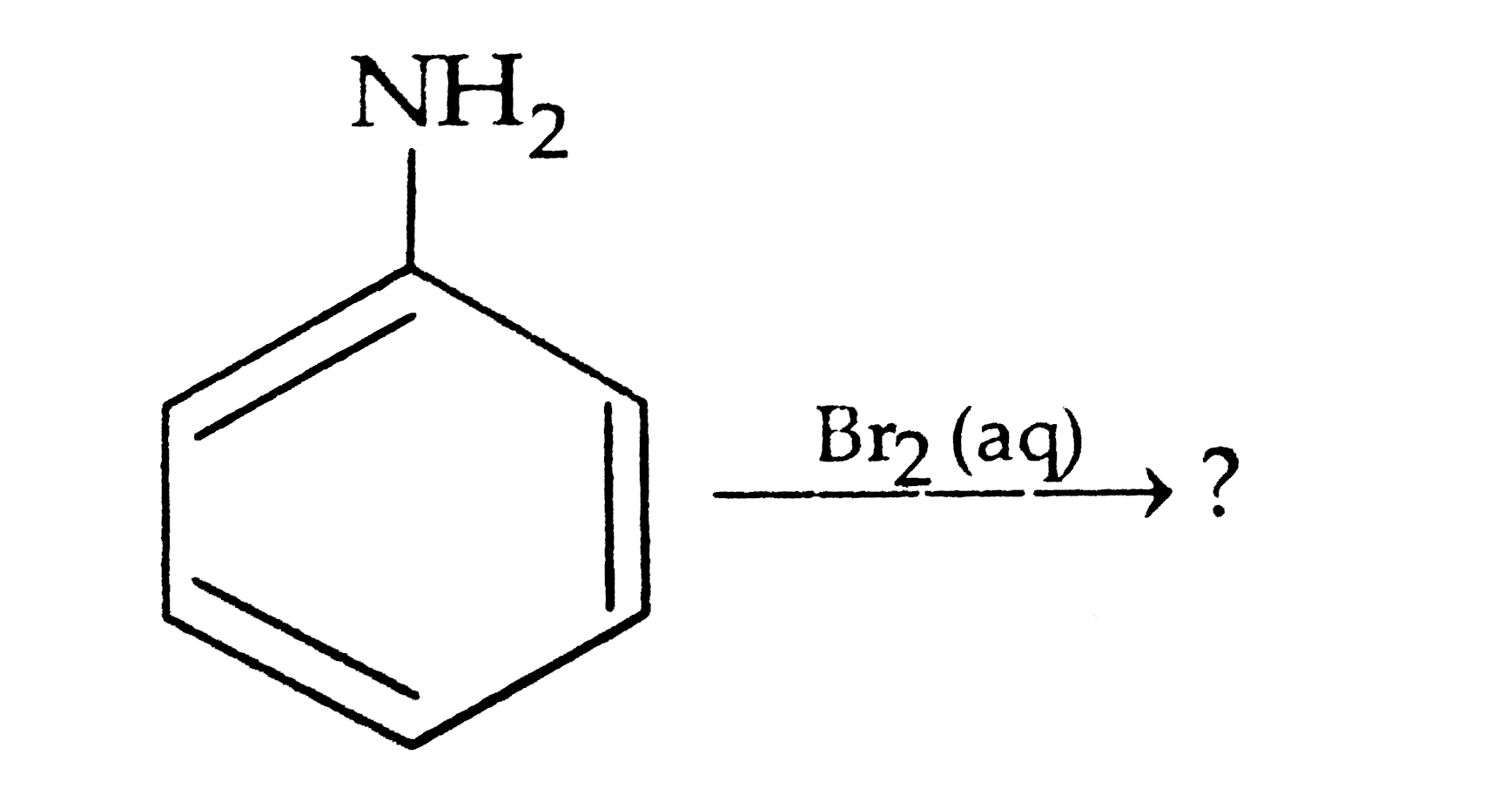
(iii) CH3−C∣∣O−NH2Br2+NaOH−−−−−−−→?
View SolutionPhCH2CH2CH3(i)CrO2Cl2/CCl4−−−−−−−−−−−−−−→(ii)h2O(A)
The product (A) is:
View SolutionC6H5N2ClSnCl2/HClA→C6H5CHO−−−−−−→(B)
The product (B) in the above sequence of reaction is
View SolutionWrite the main products of the following reactions:
(i) C6H5N+2Cl−H3PO2+H2O−−−−−−−−→?

(iii) CH3−C∣∣O−NH2Br2+NaOH−−−−−−−→?
View SolutionC6H5N2Cl+HBF4Δ−−→C6H5F+N2+BF3 . The reaction is called
View SolutionC2H5Cl + KCN →X Hydroysis−−−−−→Y, 'X' and 'Y' are:
View SolutionIdentify the total number of atoms in product Q in the given sequence of reaction C6H5NH2NaNO2+HCl−−−−−−−−→XCu2(CN)2−−−−−−→YH2O/H+−−−−−→ZNaOH/CaO,Δ−−−−−−−−−→PCl2hv(Excess)−−−−−−−→Q
View Solution
