Which one is most reactive towards nucleophilic addition reaction?
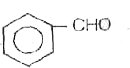
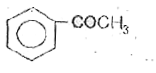
The correct Answer is:D
To determine which compound is most reactive towards nucleophilic addition reactions, we need to analyze the given compounds based on their functional groups and the presence of electron-withdrawing or electron-releasing groups.
1. Understanding Nucleophilic Addition Reactions:
Nucleophilic addition reactions involve the attack of a nucleophile on a carbon atom that is electrophilic (positively charged or partially positive). The reactivity of carbonyl compounds (like aldehydes and ketones) towards nucleophiles is influenced by the electron density on the carbonyl carbon.
2. Identifying the Compounds:
Let's denote the compounds as follows:
- A: Benzaldehyde (C6H5CHO)
- B: Acetophenone (C6H5COCH3)
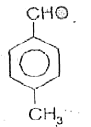
- C: p-Tolualdehyde (C6H4(CH3)CHO)
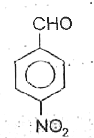
- D: p-Nitrobenzaldehyde (C6H4(NO2)CHO)
3. Analyzing Compound A (Benzaldehyde):
- Benzaldehyde has a carbonyl group (C=O) with no additional electron-withdrawing or electron-releasing groups.
- It is moderately reactive due to the presence of the aldehyde group.
4. Analyzing Compound B (Acetophenone):
- Acetophenone is a ketone and has a carbonyl group.
- Ketones are generally less reactive than aldehydes towards nucleophilic addition due to steric hindrance and the presence of an alkyl group, which is electron-releasing.
5. Analyzing Compound C (p-Tolualdehyde):
- p-Tolualdehyde has a methyl group (CH3) at the para position, which is an electron-releasing group.
- The presence of the methyl group decreases the electrophilicity of the carbonyl carbon, making it less reactive towards nucleophiles.
6. Analyzing Compound D (p-Nitrobenzaldehyde):
- p-Nitrobenzaldehyde has a nitro group (NO2) at the para position, which is a strong electron-withdrawing group.
- The electron-withdrawing nature of the nitro group increases the positive character of the carbonyl carbon, making it more reactive towards nucleophiles.
7. Comparing Reactivity:
- Between compounds A, C, and D, compound D (p-Nitrobenzaldehyde) is the most reactive due to the presence of the electron-withdrawing nitro group, which enhances the electrophilicity of the carbonyl carbon.
- Compound A (Benzaldehyde) is more reactive than compound C (p-Tolualdehyde) but less reactive than compound D.
Conclusion:
The compound that is most reactive towards nucleophilic addition reactions is D: p-Nitrobenzaldehyde.
Nucleophilic addition reactions involve the attack of a nucleophile on a carbon atom that is electrophilic (positively charged or partially positive). The reactivity of carbonyl compounds (like aldehydes and ketones) towards nucleophiles is influenced by the electron density on the carbonyl carbon.
Let's denote the compounds as follows:
- A: Benzaldehyde (C6H5CHO)
- B: Acetophenone (C6H5COCH3)
- C: p-Tolualdehyde (C6H4(CH3)CHO)
- D: p-Nitrobenzaldehyde (C6H4(NO2)CHO)
- Benzaldehyde has a carbonyl group (C=O) with no additional electron-withdrawing or electron-releasing groups.
- It is moderately reactive due to the presence of the aldehyde group.
- Acetophenone is a ketone and has a carbonyl group.
- Ketones are generally less reactive than aldehydes towards nucleophilic addition due to steric hindrance and the presence of an alkyl group, which is electron-releasing.
- p-Tolualdehyde has a methyl group (CH3) at the para position, which is an electron-releasing group.
- The presence of the methyl group decreases the electrophilicity of the carbonyl carbon, making it less reactive towards nucleophiles.
- p-Nitrobenzaldehyde has a nitro group (NO2) at the para position, which is a strong electron-withdrawing group.
- The electron-withdrawing nature of the nitro group increases the positive character of the carbonyl carbon, making it more reactive towards nucleophiles.
- Between compounds A, C, and D, compound D (p-Nitrobenzaldehyde) is the most reactive due to the presence of the electron-withdrawing nitro group, which enhances the electrophilicity of the carbonyl carbon.
- Compound A (Benzaldehyde) is more reactive than compound C (p-Tolualdehyde) but less reactive than compound D.
The compound that is most reactive towards nucleophilic addition reactions is D: p-Nitrobenzaldehyde.
Similar Questions
Knowledge Check
Which of the following compounds is most reactive towards nucleophilic addition reactions ?
ACH3−O∣∣C−HBCH3−O∣∣C−CH3C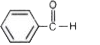 D
D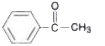
Which of the following compounds is most reactive towards nucleophilic addition reactions ?
ACH3−O∣∣C−HBCH3−O∣∣C−CH3C D
D
Which of the following compounds is most reactive towards nucleophilic addition reactions ?
ACH3−O∣∣C−HBCH3−O∣∣C−CH3C D
D
Which of the following compounds is most reactive towards nucleophilic addition reactions ?
View SolutionWhich carbonyl group of the given compound is most reactive for nucleophilic addition reaction?
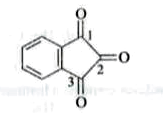
View SolutionWhich of the following alkenes is most reactive towards electrophilic addition reaction?
View SolutionWhich one of the following is most reactive towards nucleophilic substitution reaction ?
View SolutionWhich ofthe following is most reactive towards nucleophilic substitution reaction ?
View Solution
