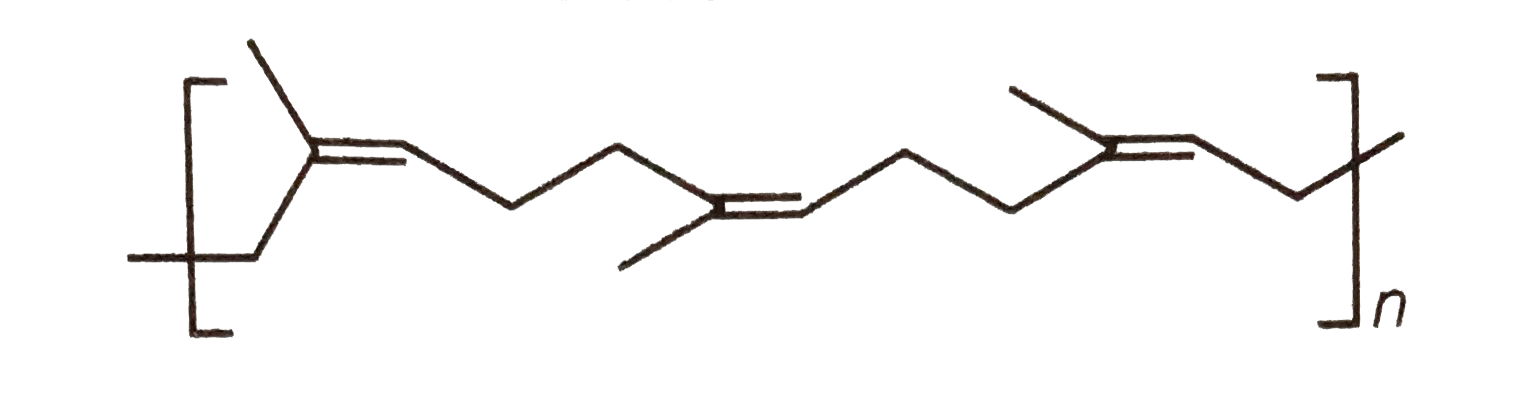
Identify the co-polymer
Buna-N
Neoprene
Natural rubber
All of these
The correct Answer is:A
To identify the co-polymer from the given options, we can follow these steps:
1. Understanding Co-polymers:
- A co-polymer is a polymer that is made from two different types of monomeric units.
2. Identifying the Monomeric Units:
- We need to look for the options provided and identify if they consist of two different monomers.
3. Analyzing the Given Options:
- Option A: Bunion (1,3-butadiene and acrylonitrile)
- Option B: Neoprene (made from chloroprene)
- Option C: Natural rubber (made from isoprene)
- Option D: Not specified in the transcript.
4. Determining the Composition of Each Option:
- Bunion:
- Composed of 1,3-butadiene (CH2=CH-CH=CH2) and acrylonitrile (CH2=CH-CN).
- Since it consists of two different monomeric units, it qualifies as a co-polymer.
- Neoprene:
- Made from chloroprene (CH2=CCl-CH=CH2), which is a homopolymer, not a co-polymer.
- Natural Rubber:
- Made from isoprene (CH2=C(CH3)-CH=CH2), which is also a homopolymer.
5. Conclusion:
- Based on the analysis, the only option that qualifies as a co-polymer is Bunion (Option A), which is formed from the combination of 1,3-butadiene and acrylonitrile.
Final Answer:
The co-polymer is Bunion (Option A).
---
- A co-polymer is a polymer that is made from two different types of monomeric units.
- We need to look for the options provided and identify if they consist of two different monomers.
- Option A: Bunion (1,3-butadiene and acrylonitrile)
- Option B: Neoprene (made from chloroprene)
- Option C: Natural rubber (made from isoprene)
- Option D: Not specified in the transcript.
- Bunion:
- Composed of 1,3-butadiene (CH2=CH-CH=CH2) and acrylonitrile (CH2=CH-CN).
- Since it consists of two different monomeric units, it qualifies as a co-polymer.
- Neoprene:
- Made from chloroprene (CH2=CCl-CH=CH2), which is a homopolymer, not a co-polymer.
- Natural Rubber:
- Made from isoprene (CH2=C(CH3)-CH=CH2), which is also a homopolymer.
- Based on the analysis, the only option that qualifies as a co-polymer is Bunion (Option A), which is formed from the combination of 1,3-butadiene and acrylonitrile.
The co-polymer is Bunion (Option A).
टॉपर्स ने हल किए ये सवाल
- POLYMERSBook:AAKASH INSTITUTE ENGLISHChapter:POLYMERSExercise:ASSIGNMENT (SECTION -A)
Explore 44 Videos
- POLYMERSBook:AAKASH INSTITUTE ENGLISHChapter:POLYMERSExercise:ASSIGNMENT (SECTION -B)
Explore 20 Videos
- POLYMERSBook:AAKASH INSTITUTE ENGLISHChapter:POLYMERSExercise:TRY YOUR SELF
Explore 7 Videos
- ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESBook:AAKASH INSTITUTE ENGLISHChapter:ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESExercise:TRY YOURSELF
Explore 28 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBook:AAKASH INSTITUTE ENGLISHChapter:PRINCIPLES OF QUALITATIVE ANALYSISExercise:Assignment (SECTION H)
Explore 9 Videos
समान प्रश्न
ज्ञान जांच
Identify the type of polymer
(i) −A−A−A−A−A−A−
(ii) −A−B−B−A−A−A−B−A−A(i) Homopolymer, (ii) CopolymerB(i) Natural polymer (ii) Synthetic polymerC(i) Linear polymer , (ii) Branched polmerD(i) Fibre , (ii) ElastomerIdentify the addtion polymer that would be produced from 2-chloro-3 butene.
A−Cl∣C∣CH3−H∣C∣CH3−Cl∣C∣CH3−H∣C∣CH3−Cl∣C∣CH3−H∣C∣CH3−B−Cl∣C∣CH3−Cl∣C∣CH3−Cl∣C∣CH3−Cl∣C∣CH3−Cl∣C∣CH3−Cl∣C∣CH3−C−C∣CH3=C∣CH3−C∣CH3=C∣CH3−C∣CH3=C∣CH3−D−CH3∣C∣CH3−Cl∣C∣H−CH3∣C∣CH3−Cl∣C∣H−CH3∣C∣CH3−Cl∣C∣H−
(i) What is the role of Sulphur in the vulcanization of rubber ?
(ii) Identify the monomers in the following polymer :
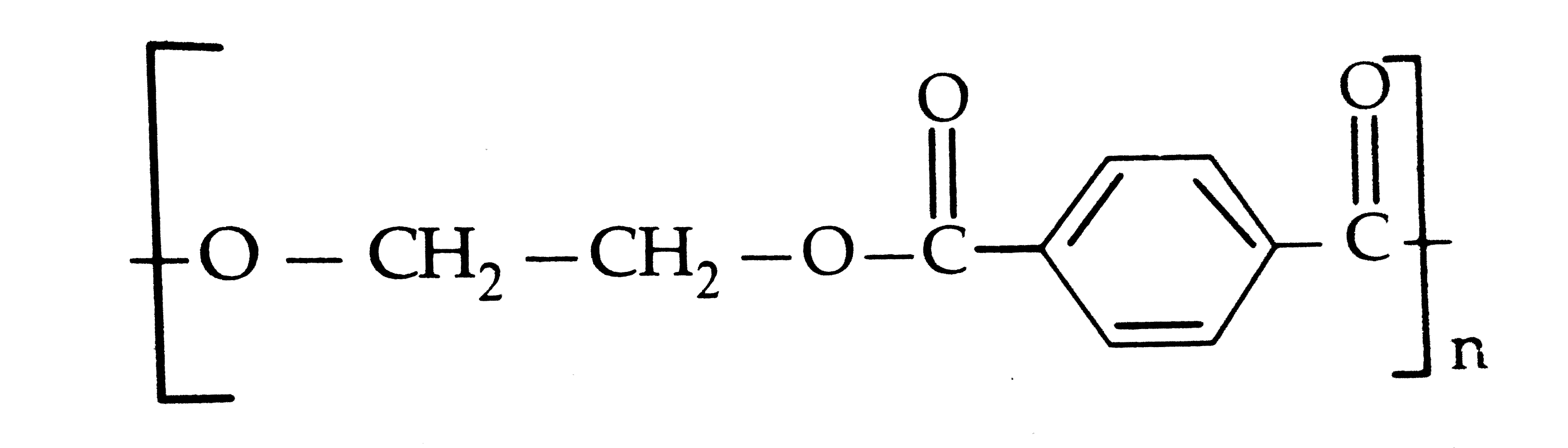
(ii) Arrange the following polymers in the increasing order of their intermolecular forces : Terylene, Polyethene, Neoprene.
View SolutionAmong the following polymer, identify compolymer ?
View SolutionIdentify the type of polymer
-A-A-A-A-A-A-
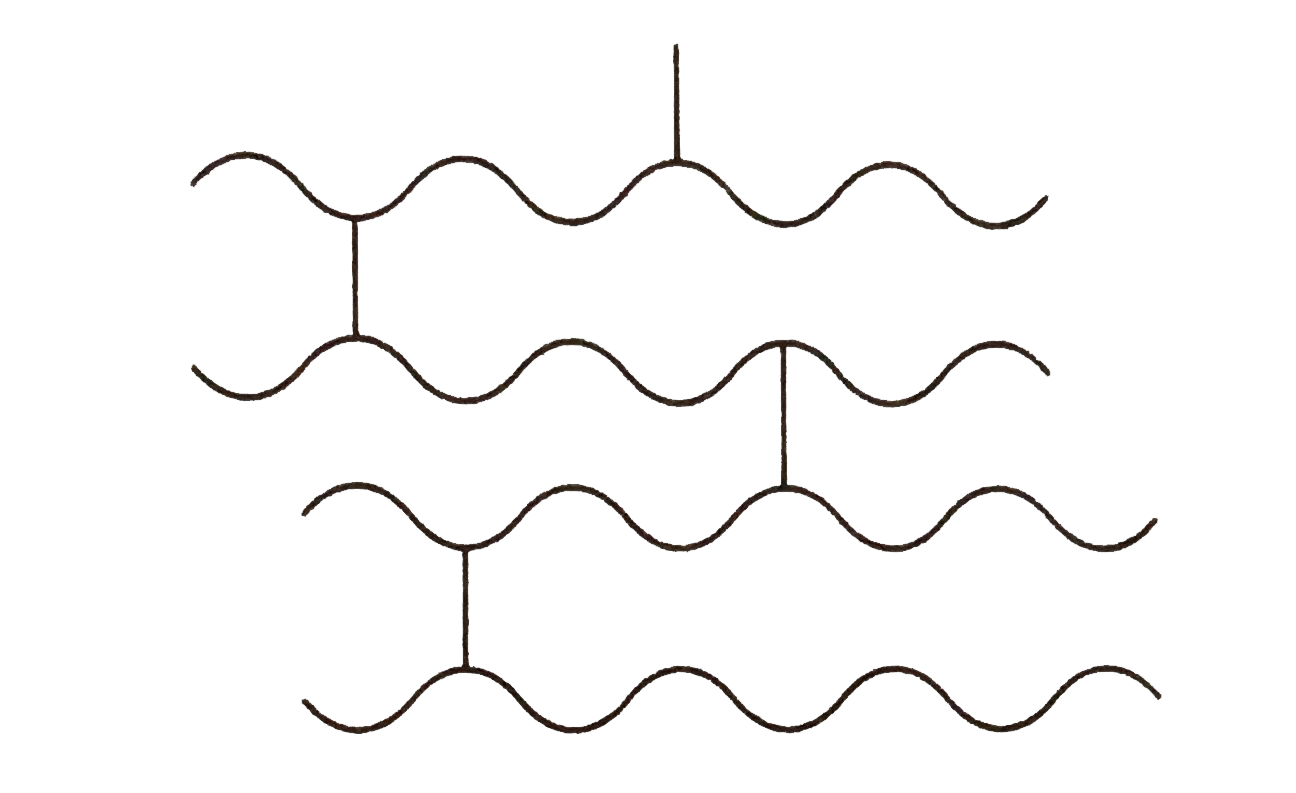
View SolutionIdentify the type of polymer given in the following figure
View SolutionIdentify the type of polymer
-A-B-B-A-A-A-B-A-
View SolutionIdentify the correct statement the fundamental period of f(x)=cos(sinx)+cos(cosx) is π
View Solution