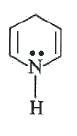Which one is not aromatic compound ?

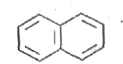
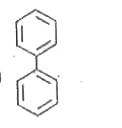

The correct Answer is:4
To determine which compound is not aromatic, we need to apply the criteria for aromaticity. An aromatic compound must satisfy the following conditions:
1. Planarity: The compound must be planar, meaning that all atoms in the ring must lie in the same plane.
2. Cyclic Structure: The compound must be cyclic, meaning it forms a ring.
3. Hückel's Rule: The compound must have a specific number of π (pi) electrons, which follows Hückel's rule: 4n+2 π electrons, where n is a non-negative integer (0, 1, 2, ...).
Now, let's analyze the given compounds one by one:
Step 1: Analyze the first compound
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
Assuming the first compound has 2 π electrons:
- It is cyclic and planar.
- It has 2 π electrons, which fits Hückel's rule when n=0 (since 4(0)+2=2).
- Conclusion: This compound is aromatic.
Step 2: Analyze the second compound (Naphthalene)
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
Naphthalene has 10 π electrons:
- It is cyclic and planar.
- It has 10 π electrons, which fits Hückel's rule when n=2 (since 4(2)+2=10).
- Conclusion: This compound is aromatic.
Step 3: Analyze the third compound (Biphenyl)
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
Biphenyl consists of two benzene rings:
- Each benzene ring has 6 π electrons, so biphenyl has a total of 12 π electrons.
- It is planar and cyclic.
- It has 12 π electrons, which fits Hückel's rule when n=3 (since 4(3)+2=14 does not fit, but each ring is aromatic).
- Conclusion: This compound is aromatic.
Step 4: Analyze the fourth compound
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
Assuming this compound has 4 π electrons:
- It is cyclic and planar.
- It has 4 π electrons, which does not satisfy Hückel's rule (since n cannot be a non-negative integer for 4 π electrons).
- Conclusion: This compound is not aromatic.
Final Answer
The compound that is not aromatic is the fourth compound.
---
2. Cyclic Structure: The compound must be cyclic, meaning it forms a ring.
3. Hückel's Rule: The compound must have a specific number of π (pi) electrons, which follows Hückel's rule: 4n+2 π electrons, where n is a non-negative integer (0, 1, 2, ...).
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
- It is cyclic and planar.
- It has 2 π electrons, which fits Hückel's rule when n=0 (since 4(0)+2=2).
- Conclusion: This compound is aromatic.
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
- It is cyclic and planar.
- It has 10 π electrons, which fits Hückel's rule when n=2 (since 4(2)+2=10).
- Conclusion: This compound is aromatic.
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
- Each benzene ring has 6 π electrons, so biphenyl has a total of 12 π electrons.
- It is planar and cyclic.
- It has 12 π electrons, which fits Hückel's rule when n=3 (since 4(3)+2=14 does not fit, but each ring is aromatic).
- Conclusion: This compound is aromatic.
- Structure: Check if it is cyclic and planar.
- Hybridization: Each carbon atom should be sp² hybridized.
- Pi Electrons: Count the number of π electrons.
- It is cyclic and planar.
- It has 4 π electrons, which does not satisfy Hückel's rule (since n cannot be a non-negative integer for 4 π electrons).
- Conclusion: This compound is not aromatic.
The compound that is not aromatic is the fourth compound.
Topper's Solved these Questions
- HYDROCARBONSBook:AAKASH INSTITUTE ENGLISHChapter:HYDROCARBONSExercise:Assignment(Section - B) (Obejctive type question)
Explore 5 Videos
- HYDROCARBONSBook:AAKASH INSTITUTE ENGLISHChapter:HYDROCARBONSExercise:Assignment(Section - C) (Previous Years Questions)
Explore 60 Videos
- HYDROCARBONSBook:AAKASH INSTITUTE ENGLISHChapter:HYDROCARBONSExercise:Exercise
Explore 33 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTE ENGLISHChapter:HALOALKANES AND HALOARENES Exercise:ASSIGNMENT SECTION -D
Explore 15 Videos
- HYDROGENBook:AAKASH INSTITUTE ENGLISHChapter:HYDROGENExercise:ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)
Explore 15 Videos
Similar Questions
Knowledge Check
Which of the following is not an aromatic compound ?
A B
B C
C D
D
Which of the following is not an aromatic compound ?
A B
B C
C D
D
For any compound ot be aromatic, compound should follow a given set of rule known as Huckel's rule.
According to Huckel's rule of aromaticity :
(a) compound should be cyclic
(b) compound should be planar and conjugated.
(c ) compound should have (4n+2)πe−
where n = 0, 1, 2, 3.. integer number.
Which of the following is not an aromatic compound ?A B
B C
C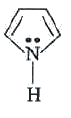 D
D